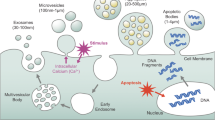
Abstract
Extracellular vesicles (EVs) are membrane-bound vesicles released into the extracellular space by almost all types of cells. EVs can cross the physiological barriers, and a variety of biological fluids are enriched in them. EVs are a heterogeneous population of vesicles, including exosomes, microvesicles, and apoptotic bodies. The different subpopulations of vesicles can be differentiated by size and origin, in which exosomes (~100 nm and from endocytic origin) are the most studied so far. EVs have essential roles in cell-to-cell communication and are critical modulators of immune response under normal and pathological conditions. Pregnancy is a unique situation of immune-modulation in which the maternal immune system protects the fetus from allogenic rejection and maintains the immunosurveillance. The placenta is a vital organ that performs a multitude of functions to support the pregnancy. The EVs derived from the human placenta have crucial roles in regulating the maternal immune response for successful pregnancy outcome. Placenta-derived vesicles perform a myriad of functions like suppression of immune reaction to the developing fetus and establishment and maintenance of a systemic inflammatory response to combat infectious intruders. A fine-tuning of these mechanisms is quintessential for successful completion of pregnancy and healthy outcome for mother and fetus. Dysregulation in the mechanisms mentioned above can lead to several pregnancy disorders. In this review, we summarize the current literature regarding the critical roles played by the EVs in immunomodulation during pregnancy with particular attention to the placenta-derived exosomes.


Similar content being viewed by others
References
Abumaree MH, Chamley LW, Badri M, El-Muzaini MF (2012) Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J Reprod Immunol 94(2):131–141
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345. https://doi.org/10.1038/nbt.1807
Alam SMK, Jasti S, Kshirsagar SK, Tannetta DS, Dragovic RA, Redman CW, Sargent IL, Hodes HC, Nauser TL, Fortes T, Filler AM, Behan K, Martin DR, Fields TA, Petroff BK, Petroff MG (2018) Trophoblast glycoprotein (TPGB/5T4) in human placenta: expression, regulation, and presence in extracellular microvesicles and exosomes. Reprod Sci 25(2):185–197. https://doi.org/10.1177/1933719117707053.
An T, Qin S, Xu Y, Tang Y, Huang Y, Situ B et al (2015) Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracell Vesicles 4:27522. https://doi.org/10.3402/jev.v4.27522
Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P et al (2002) Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360(9329):295–305. https://doi.org/10.1016/s0140-6736(02)09552-1
Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A (2009) Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127(1):26–39. https://doi.org/10.1111/j.1365-2567.2008.03019.x
Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT (2010) Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res 70(2):481–489. https://doi.org/10.1158/0008-5472.can-09-1688
Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD (2011a) Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol 65(1):65–77. https://doi.org/10.1111/j.1600-0897.2010.00880.x
Atay S, Gercel-Taylor C, Taylor DD (2011b) Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1beta production by macrophages. Am J Reprod Immunol 66(4):259–269. https://doi.org/10.1111/j.1600-0897.2011.00995.x
Bai J, Gao Z, Li X, Dong L, Han W, Nie J (2017) Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget 8(66):110693–110707. https://doi.org/10.18632/oncotarget.22690
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A et al (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14(7):677–685. https://doi.org/10.1038/ncb2502
Billington WD (2003) The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol 60(1):1–11
Bloemendal S, Kuck U (2013) Cell-to-cell communication in plants, animals, and fungi: a comparative review. Naturwissenschaften 100(1):3–19. https://doi.org/10.1007/s00114-012-0988-z
Carp H, Dardik R, Lubetsky A, Salomon O, Eskaraev R, Rosenthal E, Inbal A (2004) Prevalence of circulating procoagulant microparticles in women with recurrent miscarriage: a case-controlled study. Hum Reprod 19(1):191–195
Chamley LW, Chen Q, Ding J, Stone PR, Abumaree M (2011) Trophoblast deportation: just a waste disposal system or antigen sharing? J Reprod Immunol 88(2):99–105. https://doi.org/10.1016/j.jri.2011.01.002
Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J (2004) TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol 134(2):93–119. https://doi.org/10.1159/000074300
Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledée N (2010) Tolerance to the foetal allograft? Am J Reprod Immunol 63(6):624–636
Chaput N, Théry C (2011) Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 33(5):419–440. https://doi.org/10.1007/s00281-010-0233-9
Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P et al (2013) Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126(Pt 24):5553–5565. https://doi.org/10.1242/jcs.128868
Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y et al (2013) Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A 110(29):12048–12053. https://doi.org/10.1073/pnas.1304718110
Donker RB, Mouillet JF, Chu T, Hubel CA, Stolz DB, Morelli AE, Sadovsky Y (2012) The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. MHR: Basic Sci Reprod Med 18(8):417–424. https://doi.org/10.1093/molehr/gas013
Elfeky O, Longo S, Lai A, Rice GE, Salomon C (2017) Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta 50:60–69. https://doi.org/10.1016/j.placenta.2016.12.020
Escudero CA, Herlitz K, Troncoso F, Acurio J, Aguayo C, Roberts JM et al (2016) Role of extracellular vesicles and microRNAs on dysfunctional angiogenesis during preeclamptic pregnancies. Front Physiol 7:98. https://doi.org/10.3389/fphys.2016.00098
Frangsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva-Nilsson L (2005) Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod 11(1):35–41. https://doi.org/10.1093/molehr/gah129
Gasser O, Schifferli JA (2004) Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104(8):2543–2548. https://doi.org/10.1182/blood-2004-01-0361
Gatson NN, Williams JL, Powell ND, McClain MA, Hennon TR, Robbins PD, Whitacre CC (2011) Induction of pregnancy during established EAE halts progression of CNS autoimmune injury via pregnancy-specific serum factors. J Neuroimmunol 230(1–2):105–113. https://doi.org/10.1016/j.jneuroim.2010.09.010
Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW (2007) Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol 178(9):5949–5956
Gohner C, Weber M, Tannetta DS, Groten T, Plosch T, Faas MM et al (2015) A new enzyme-linked sorbent assay (ELSA) to quantify syncytiotrophoblast extracellular vesicles in biological fluids. Am J Reprod Immunol 73(6):582–588. https://doi.org/10.1111/aji.12367
Gohner C, Plosch T, Faas MM (2017) Immune-modulatory effects of syncytiotrophoblast extracellular vesicles in pregnancy and preeclampsia. Placenta 60:S41–S51. https://doi.org/10.1016/j.placenta.2017.06.004
Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S (2005a) Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol 66(11):1146–1154. https://doi.org/10.1016/j.humimm.2005.11.003
Gupta AK, Rusterholz C, Holzgreve W, Hahn S (2005b) Syncytiotrophoblast micro-particles do not induce apoptosis in peripheral T lymphocytes, but differ in their activity depending on the mode of preparation. J Reprod Immunol 68(1):15–26. https://doi.org/10.1016/j.jri.2005.05.003
Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, Mincheva-Nilsson L (2009) Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol 183(1):340–351. https://doi.org/10.4049/jimmunol.0803477
Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA (1999) Ectosomes released by human neutrophils are specialized functional units. J Immunol 163(8):4564–4573
Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, Abrahams VM (2012a) Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology 136(2):184–191. https://doi.org/10.1111/j.1365-2567.2012.03568.x
Holder BS, Tower CL, Jones CJ, Aplin JD, Abrahams VM (2012b) Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol Reprod 86(4):103. https://doi.org/10.1095/biolreprod.111.097014
Holder B, Jones T, Sancho Shimizu V, Rice TF, Donaldson B, Bouqueau M et al (2016) Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal–placental messaging. Traffic 17(2):168–178. https://doi.org/10.1111/tra.12352
Kambe S, Yoshitake H, Yuge K, Ishida Y, Ali MM, Takizawa T et al (2014) Human exosomal placenta-associated miR-517a-3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biol Reprod 91(5):129. https://doi.org/10.1095/biolreprod.114.121616
Knight M, Redman CW, Linton EA, Sargent IL (1998) Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol 105:632–640. https://doi.org/10.1111/j.1471-0528.1998.tb10178.x
Kohli S, Isermann B (2017) Placental hemostasis and sterile inflammation: new insights into gestational vascular disease. Thromb Res 151:S30–S33. https://doi.org/10.1016/S0049-3848(17)30063-4
Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM et al (2016) Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 128(17):2153–2164. https://doi.org/10.1182/blood-2016-03-705434
Kowal J, Tkach M, Thery C (2014) Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29:116–125. https://doi.org/10.1016/j.ceb.2014.05.004
Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M et al (2012) Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 33(12):982–990. https://doi.org/10.1016/j.placenta.2012.10.005
Laresgoiti-Servitje E (2013) A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol 94(2):247–257. https://doi.org/10.1189/jlb.1112603
Lässer C, Seyed Alikhani V, Ekström K, Eldh M, Torregrosa Paredes P, Bossios A et al (2011) Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med 9:9. https://doi.org/10.1186/1479-5876-9-9
Laude I, Rongieres-Bertrand C, Boyer-Neumann C, Wolf M, Mairovitz V, Hugel B et al (2001) Circulating procoagulant microparticles in women with unexplained pregnancy loss: a new insight. Thromb Haemost 85(1):18–21
Le Bouteiller P, Bensussan A (2017) Up-and-down immunity of pregnancy in humans. F1000Research 6:1216. https://doi.org/10.12688/f1000research.11690.1
Lee SM, Romero R, Lee YJ, Park IS, Park CW, Yoon BH (2012) Systemic inflammatory stimulation by microparticles derived from hypoxic trophoblast as a model for inflammatory response in preeclampsia. Am J Obstet Gynecol 207(4):337.e331–337.e338. https://doi.org/10.1016/j.ajog.2012.06.047
Lissauer D, Eldershaw SA, Inman CF, Coomarasamy A, Moss PA, Kilby MD (2015) Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur J Immunol 45(10):2858–2872. https://doi.org/10.1002/eji.201445404
Lok CA, Van Der Post JA, Sargent IL, Hau CM, Sturk A, Boer K, Nieuwland R (2008) Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy 27(4):344–360. https://doi.org/10.1080/10641950801955733
Lok CA, Jebbink J, Nieuwland R, Faas MM, Boer K, Sturk A, Van Der Post JA (2009) Leukocyte activation and circulating leukocyte-derived microparticles in preeclampsia. Am J Reprod Immunol 61(5):346–359. https://doi.org/10.1111/j.1600-0897.2009.00701.x
Lok CA, Snijder KS, Nieuwland R, Van Der Post JA, de Vos P, Faas MM (2012) Microparticles of pregnant women and preeclamptic patients activate endothelial cells in the presence of monocytes. Am J Reprod Immunol 67(3):206–215. https://doi.org/10.1111/j.1600-0897.2011.01079.x
Maas SLN, Breakefield XO, Weaver AM (2017) Extracellular Vesicles: unique Intercellular Delivery Vehicles. Trends Cell Biol 27(3):172–188. https://doi.org/10.1016/j.tcb.2016.11.003
Makris V, Daniilidis A, Koiou A, Balaouras D, Fotinakis I, Spathopoulou S et al (2015) Microparticles hyperactivity in a case of intrauterine growth restriction. Clin Exp Obstet Gynecol 42(2):231–233
Marsh M, van Meer G (2008) Cell biology. No ESCRTs for exosomes. Science 319(5867):1191–1192. https://doi.org/10.1126/science.1155750
Messerli M, May K, Hansson SR, Schneider H, Holzgreve W, Hahn S, Rusterholz C (2009) Feto-maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes. Placenta 31:106–112. https://doi.org/10.1016/j.placenta.2009.11.011
Mikhailova VA, Ovchinnikova OM, Zainulina MS, Sokolov DI, Sel’kov SA (2014) Detection of microparticles of leukocytic origin in the peripheral blood in normal pregnancy and preeclampsia. Bull Exp Biol Med 157(6):751–756. https://doi.org/10.1007/s10517-014-2659-x
Mincheva-Nilsson L, Baranov V (2014) Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol 72(5):440–457. https://doi.org/10.1111/aji.12311
Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE et al (2015) Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol 213(4, Supplement):S173–S181. https://doi.org/10.1016/j.ajog.2015.07.001
Moffett-King A (2002) Natural killer cells and pregnancy. Nat Rev Immunol 2(9):656–663. https://doi.org/10.1038/nri886
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM et al (2012) Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119(3):756–766. https://doi.org/10.1182/blood-2011-02-338004
Mor G, Cardenas I (2010) The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 63(6):425–433. https://doi.org/10.1111/j.1600-0897.2010.00836.x
Moro L, Bardaji A, Macete E, Barrios D, Morales-Prieto DM, Espana C et al (2016) Placental microparticles and MicroRNAs in pregnant women with plasmodium falciparum or HIV infection. PLoS One 11(1):e0146361. https://doi.org/10.1371/journal.pone.0146361
Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3. https://doi.org/10.3402/jev.v3.24641
Nardi Fda S, Michelon TF, Neumann J, Manvailer LF, Wagner B, Horn PA et al (2016) High levels of circulating extracellular vesicles with altered expression and function during pregnancy. Immunobiology 221(7):753–760. https://doi.org/10.1016/j.imbio.2016.03.001
Ospina-Prieto S, Chaiwangyen W, Herrmann J, Groten T, Schleussner E, Markert UR, Morales-Prieto DM (2016) MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl Res 172:61–72. https://doi.org/10.1016/j.trsl.2016.02.012
Ouyang Y, Bayer A, Chu T, Tyurin VA, Kagan VE, Morelli AE et al (2016) Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta 47:86–95. https://doi.org/10.1016/j.placenta.2016.09.008
Pap E, Pallinger E, Falus A, Kiss AA, Kittel A, Kovacs P, Buzas EI (2008) T lymphocytes are targets for platelet- and trophoblast-derived microvesicles during pregnancy. Placenta 29(9):826–832. https://doi.org/10.1016/j.placenta.2008.06.006
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A et al (2009) Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 284(49):34211–34222. https://doi.org/10.1074/jbc.M109.041152
Piccinni MP (2002) T-cell cytokines in pregnancy. Am J Reprod Immunol 47(5):289–294
Radu CM, Campello E, Spiezia L, Dhima S, Visentin S, Gavasso S et al (2015) Origin and levels of circulating microparticles in normal pregnancy: a longitudinal observation in healthy women. Scand J Clin Lab Invest 75(6):487–495. https://doi.org/10.3109/00365513.2015.1052551
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183(3):1161–1172
Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308(5728):1592–1594. https://doi.org/10.1126/science.1111726
Redman CWG, Sargent IL (2010) Immunology of pre-eclampsia. Am J Reprod Immunol 63(6):534–543
Rice GE, Scholz-Romero K, Sweeney E, Peiris H, Kobayashi M, Duncombe G et al (2015) The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. J Clin Endocrinol Metab 100(10):E1280–E1288. https://doi.org/10.1210/jc.2015-2270
Sabapatha A, Gercel-Taylor C, Taylor DD (2006) Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol 56(5–6):345–355. https://doi.org/10.1111/j.1600-0897.2006.00435.x
Saito S, Miyazaki S, Sasaki Y (2006) Th1/Th2 balance of the implantation site in humans. In: Mor G. (eds) Immunol Pregnancy. Medical Intelligence Unit. Springer, New York, NY.https://doi.org/10.1007/0-387-34944-8_4
Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P et al (2016) Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes 65(3):598–609. https://doi.org/10.2337/db15-0966
Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C (2014) Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 12:204. https://doi.org/10.1186/1479-5876-12-204
Schumacher A, Heinze K, Witte J, Poloski E, Linzke N, Woidacki K, Zenclussen AC (2013) Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol 190(6):2650–2658. https://doi.org/10.4049/jimmunol.1202698
Segura E, Guérin C, Hogg N, Amigorena S, Théry C (2007) CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol 179(3):1489–1496. https://doi.org/10.4049/jimmunol.179.3.1489
Southcombe J, Tannetta D, Redman C, Sargent I (2011) The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One 6:e20245. https://doi.org/10.1371/journal.pone.0020245
Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L (2013) Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol 191(11):5515–5523. https://doi.org/10.4049/jimmunol.1301885
Stuffers S, Sem Wegner C, Stenmark H, Brech A (2009) Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10(7):925–937. https://doi.org/10.1111/j.1600-0854.2009.00920.x
Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL (2013) Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One 8(2):e56754. https://doi.org/10.1371/journal.pone.0056754
Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J (2014) Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol Immunol 11(6):548–563. https://doi.org/10.1038/cmi.2014.42
Tannetta DS, Hunt K, Jones CI, Davidson N, Coxon CH, Ferguson D et al (2015) Syncytiotrophoblast extracellular vesicles from pre-eclampsia placentas differentially affect platelet function. PLoS One 10(11):e0142538. https://doi.org/10.1371/journal.pone.0142538
Taylor DD, Akyol S, Gercel-Taylor C (2006) Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 176(3):1534–1542
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579. https://doi.org/10.1038/nri855
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9(8):581–593
Thibault G, Degenne D, Girard AC, Guillaumin JM, Lacord M, Bardos P (1991) The inhibitory effect of human syncytiotrophoblast plasma membrane vesicles on in vitro lymphocyte proliferation is associated with reduced interleukin 2 receptor expression. Cell Immunol 138(1):165–174
Tilburgs T, Strominger JL (2013) CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol 69(4):395–407. https://doi.org/10.1111/aji.12094
Tolosa JM, Schjenken JE, Clifton VL, Vargas A, Barbeau B, Lowry P et al (2012) The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 33(11):933–941. https://doi.org/10.1016/j.placenta.2012.08.004
Tong M, Chamley LW (2015) Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb Perspect Med 5(3):a023028. https://doi.org/10.1101/cshperspect.a023028
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319(5867):1244–1247. https://doi.org/10.1126/science.1153124
Truong G, Guanzon D, Kinhal V, Elfeky O, Lai A, Longo S et al (2017) Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells—liquid biopsies for monitoring complications of pregnancy. PLoS One 12(3):e0174514. https://doi.org/10.1371/journal.pone.0174514
Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N et al (2013) Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4:2980. https://doi.org/10.1038/ncomms3980
Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M (2014) Sorting it out: regulation of exosome loading. Semin Cancer Biol 28:3–13. https://doi.org/10.1016/j.semcancer.2014.04.009
Williams JL, Gatson NN, Smith KM, Almad A, McTigue DM, Whitacre CC (2013) Serum exosomes in pregnancy-associated immune modulation and neuroprotection during CNS autoimmunity. Clin Immunol 149(2):236–243. https://doi.org/10.1016/j.clim.2013.04.005
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D et al (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4(5):594–600
Funding
This study received funding from Lions Medical Research Foundation and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1170809).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is a contribution to the special issue on Extracellular Vesicles - Guest Editor: Esther Nolte-‘t Hoen
Rights and permissions
About this article
Cite this article
Nair, S., Salomon, C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin Immunopathol 40, 425–437 (2018). https://doi.org/10.1007/s00281-018-0680-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-018-0680-2