Abstract
Purpose/introduction
Glioblastoma (GB) remains incurable despite aggressive chemotherapy, radiotherapy, and surgical interventions; immunotherapies remain experimental in clinical practice. Relevant preclinical models that can accurately predict tumor response to therapy are equally challenging. This study aimed to validate the effect of the naturally occurring agent diallyl trisulfide (DATS) in human GB in relevant pre-clinical models.
Methods
Ex vivo slice culture, in vivo cell line derived orthotopic xenograft and patient-derived orthotopic xenograft (PDX) animal models of GB were utilized to assess efficacy of treatment with DATS.
Results
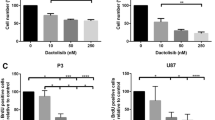
Our results showed 72-h treatments of 25 µM DATS induced cell death in ex vivo human GB slice culture. We treated U87MG orthotopic xenograft models (U87MGOX) and patient-derived orthotopic xenograft models (PDX) with daily intraperitoneal injections of DATS for 14 days. Magnetic resonance (MR) imaging of mice treated with DATS (10 mg/kg) demonstrated reduced tumor size at 5 weeks when compared with saline-treated U87MGOX and PDX controls. Hematoxylin (H&E) staining demonstrated dose-dependent reduction in gross tumor volume with decreased proliferation and decreased angiogenesis. Western blotting showed that DATS was associated with increases in histone acetylation (Ac-Histone H3/H4) and activated caspase-3 in this novel preclinical model. Histological assessment and enzyme assays showed that even the highest dose of DATS did not negatively impact hepatic function.
Conclusions
DATS may be an effective and well-tolerated therapeutic agent in preventing tumor progression and inducing apoptosis in human GB.





Similar content being viewed by others
References
Aldape K et al (2015) Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129(6):829–848
Iacob G, Dinca EB (2009) Current data and strategy in glioblastoma multiforme. J Med Life 2:386–393
Robins HI, Lassman AB, Khuntia D (2009) Therapeutic advances in malignant glioma: current status and future prospects. Neuroimaging Clin N Am 19:647–656
Lau D, Magill ST, Aghi MK (2014) Molecularly targeted therapies for recurrent glioblastoma: current and future targets. Neurosurg Focus 37(6):E15
Eyal S, Hsiao P, Unadkat JD (2009) Drug interactions at the blood-brain barrier: fact or fantasy? Pharmacol Ther 123:80–104
Neyns B, D’Haeseleer M, Rogiers A, Van de Cauter J, Chaskis C, Michotte A, Strik H (2010) The role of cytotoxic drugs in the treatment of central nervous system gliomas. Acta Neurol Belg 110:1–14
Bezecny P (2014) Histone deacetylase inhibitors in glioblastoma: pre-clinical and clinical experience. Med Oncol 31(6):985
Yin D, Ong JM, Hu J, Desmond JC, Kawamata N, Konda BM, Black KL, Koeffler HP (2007) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res 13(3):1045–1052
Chase A, Cross NC (2011) Aberrations of EZH2 in cancer. Clin Cancer Res 17(9):2613–2618
Yong RL, Tsankova NM (2015) Emerging interplay of genetics and epigenetics in gliomas: a new hope for targeted therapy. Semin Pediatr Neurol 22(1):14–22
Tsankova NM, Canoll P (2014) Advances in genetic and epigenetic analyses of gliomas: a neuropathological perspective. J Neurooncol 119(3):481–490
Pan J, Zhang L, Xu S, Cheng X, Yu H, Bao J, Lu R (2018) Induction of apoptosis inhuman papillary-thyroid-carcinoma BCPAP cells by diallyl trisulfide through activation of the MAPK signaling pathway. J Agric Food Chem 66(23):5871–5878
Choi YH (2017) Diallyl trisulfide induces apoptosis and mitotic arrest in AGS human gastric carcinoma cells through reactive oxygen species-mediated activation of AMP-activated protein kinase. Biomed Pharmacother 94:63–71
Wei Z, Shan Y, Tao L, Liu Y, Zhu Z, Liu Z, Wu Y, Chen W, Wang A, Lu Y (2017) Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1α synthesis, and decreases breast cancer metastasis. Mol Carcinog 56(10):2317–2331
Jiang XY, Zhu XS, Xu HY, Zhao ZX, Li SY, Li SZ, Cai JH, Cao JM (2017) Diallyl trisulfide suppresses tumor growth through the attenuation of Nrf2/Akt and activation of p38/JNK and potentiates cisplatin efficacy in gastric cancer treatment. Acta Pharmacol Sin 38(7):1048–1058
Liu Y, Zhu P, Wang Y, Wei Z, Tao L, Zhu Z, Sheng X, Wang S, Ruan J, Liu Z, Cao Y, Shan Y, Sun L, Wang A, Chen W, Lu Y (2015) Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK signaling pathways. PLoS One 10(4):e0123781
Xiao D, Zeng Y, Singh SV (2009) Diallyl trisulfide-induced apoptosis in human cancer cells is linked to checkpoint kinase 1-mediated mitotic arrest. Mol Carcinog 48(11):1018–1029
Singh SV, Powolny AA, Stan SD, Xiao D, Arlotti JA, Warin R, Hahm ER, Marynowski SW, Bommareddy A, Potter DM, Dhir R (2008) Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res 68(22):9503–9511
Li X, Meng Y, Xie C, Zhu J, Wang X, Li Y, Geng S, Wu J, Zhong C, Li M (2018) Diallyl trisulfide inhibits breast cancer stem cells via suppression of Wnt/β-catenin pathway. J Cell Biochem 119(5):4134–4141
Das A, Banik NL, Ray SK (2007) Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 110:1083–1095
Wallace GC IV, Haar CP, Vandergrift WA III, Giglio P, Dixon-Mah YN, Varma AK, Ray SK, Patel SJ, Banik NL, Das A (2013) Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J Neuro-oncol 42(37):13311–13314
Sun X, Guo T, He J, Zhao M, Yan M, Cui F, Deng Y (2006) Determination of the concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi 126:521–527
Das A, Cheng RR, Hilbert ML, Dixon-Moh YN, Decandio M, Vandergrift WA III, Banik NL, Lindhorst SM, Cachia D, Varma AK, Patel SJ, Giglio P (2015) Synergistic effects of crizotinib and temozolomide in experimental FIG-ROS1 fusion-positive glioblastoma. Cancer Growth Metastasis 8:51–60
Das A, McDonald DG, Dixon-Mah YN, Jacqmin DJ, Samant VN, Vandergrift WA III, Lindhorst SM, Cachia D, Varma AK, Vanek KN, Banik NL, Jenrette JM III, Raizer JJ, Giglio P, Patel SJ (2016) RIP1 and RIP3 complex regulates radiation-induced programmed necrosis in glioblastoma. Tumour Biol 37(6):7525–7534
Gemma S, Faccioli S, Chieco P, Sbraccia M, Testai E, Vittozzi L (1996) In vivo CHCl3 bioactivation, toxicokinetics, toxicity, and induced compensatory cell proliferation in B6C3F1 male mice. Toxicol Appl Pharmacol 141:394–402
Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, Oral U (2005) Liver and kidney toxicity in chronic use of opioids: an experimental long term treatment model. J Biosci 30:245–252
Entin-Meer M, Yang X, VandenBerg SR, Lamborn KR, Nudelman A, Rephaeli A, Haas-Kogan DA (2007) In vivo efficacy of a novel histone deacetylase inhibitor in combination with radiation for the treatment of gliomas. Neuro Oncol 9(2):82–88
Xiao D, Li M, Herman-Antosiewicz A, Antosiewicz J, Xiao H, Lew KL, Zeng Y, Marynowski SW, Singh SV (2006) Diallyl trisulfide inhibits angiogenic features of human umbilical vein endothelial cells by causing Akt inactivation and down-regulation of VEGF and VEGF-R2. Nutr Cancer 55(1):94–107
Funding
Completion of this project was made possible in part by the Jerry Zucker Fund for Brain Tumor Research and Funds from the Department of Neurosurgery. This work was conducted in a facility constructed with support from the National Institutes of Health, Grant Number C06 RR015455.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they have no conflict of interest.
Ethical approval
All procedures involving animals conformed to the Institutional Animal Care and Use Committee’s and the National Institute of Health’s guidelines. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the non therapeutic human study.
Rights and permissions
About this article
Cite this article
Das, A., Henderson, F., Lowe, S. et al. Single agent efficacy of the HDAC inhibitor DATS in preclinical models of glioblastoma. Cancer Chemother Pharmacol 82, 945–952 (2018). https://doi.org/10.1007/s00280-018-3684-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3684-7