Abstract
Purpose
To compare a cohort of patients with platinum-resistant recurrent ovarian cancer (PROC) treated with bevacizumab and gemcitabine (Bev–Gem) to that of patients treated only with gemcitabine (Gem).
Methods
Between 2011 and 2017, we identified the Bev–Gem and Gem PROC groups. The regimen included 1000 mg/m2 of Gem on days 1, 8, and 15, and 15 mg/m2 of Bev on day 1, every 4 weeks. Progression-free survival (PFS) and overall survival (OS) were calculated from the date of the administration of Bev–Gem or Gem until disease progression or death.
Results
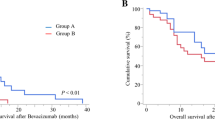
The Bev–Gem and Gem groups included 18 and 29 patients, respectively. More patients had advanced stage disease in the Bev–Gem group (p = 0.048); no other characteristics differed between the groups. The response rates [ratio of complete remission (CR) to partial remission (PR)] of Bev–Gem and Gem were 38.9 and 3.4%, respectively (p < 0.01). The clinical benefit rates [combined percentages of CR, PR, and stable disease] of the Bev–Gem and Gem groups were 88.9 and 41.4%, respectively (p = 0.04). PFS and OS of the Bev–Gem group were superior (p < 0.01, p = 0.03, respectively). Bev–Gem was the better prognostic factor of both PFS [hazard ratio (HR) 0.17, p < 0.01] and OS (HR 0.31, p = 0.01). The frequency of hematologic and non-hematologic adverse effects was similar in each group.
Conclusion
Bev–Gem regimens improved PFS and OS for PROC. Furthermore, the adverse effects of Bev–Gem were tolerable. Thus, Bev–Gem could be a candidate treatment strategy for PROC.

Similar content being viewed by others
References
Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, DeSimone CP, Ueland FR, van Nagell JR, Seamon LG (2012) Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol 120:612–618. https://doi.org/10.1097/AOG.0b013e318264f794
Pujade-Lauraine E, Alexandre J (2011) Update of randomized trials in recurrent disease. Ann Oncol 22:viii61–viii64. https://doi.org/10.1093/annonc/mdr518
Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, Wang Y, Scribner DR Jr, Marciniack M, Naumann RW, Secord AA (2007) Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 25:2811–2818. https://doi.org/10.1200/JCO.2006.09.6735
Coleman RL, Monk BJ, Sood AK, Herzog TJ (2013) Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol 10:211–224. https://doi.org/10.1038/nrclinonc.2013.5
Naumann RW, Coleman RL (2011) Management strategies for recurrent platinum-resistant ovarian cancer. Drugs 71:1397–1412. https://doi.org/10.2165/11591720-000000000-00000
Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ (2001) Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19:3312–3322. https://doi.org/10.1200/JCO.2001.19.14.3312
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stähle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM, ICON7 Investigators (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484–2496. https://doi.org/10.1056/NEJMoa1103799
Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI (2007) Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 25:5165–5171. https://doi.org/10.1200/JCO.2007.11.5345
Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D, Wenham R, McGuire W (2007) Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 25:5180–5186. https://doi.org/10.1200/JCO.2007.12.0782
Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV (2015) Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol 139:10–16. https://doi.org/10.1016/j.ygyno.2015.08.004
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray-Coquard I (2014) Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302–1308. https://doi.org/10.1200/JCO.2013.51.4489
Poveda AM, Selle F, Hilpert F, Reuss A, Savarese A, Vergote I, Witteveen P, Bamias A, Scotto N, Mitchell L, Pujade-Lauraine E (2015) Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol 33:3836–3838. https://doi.org/10.1200/JCO.2015.63.1408
Wu MD, Wang Y, Ding T, Zhou YF, Song J, Liu XH, Wei MH, Yang QH, Zhou J, Wang SH, Lv QY (2014) A retrospective clinical study of bevacizumab combined with gemcitabine or paclitaxel in the treatment of recurrent ovarian cancer. Indian J Cancer 51 Suppl 3:e103–e105. https://doi.org/10.4103/0019-509X.154096
Kawaguchi H, Terai Y, Tanabe A, Sasaki H, Takai M, Fujiwara S, Ashihara K, Tanaka Y, Tanaka T, Tsunetoh S, Kanemura M, Ohmichi M (2014) Gemcitabine as a molecular targeting agent that blocks the Akt cascade in platinum-resistant ovarian cancer. J Ovarian Res 7:38. https://doi.org/10.1186/1757-2215-7-38
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15:232–239. https://doi.org/10.1016/j.ccr.2009.01.021
Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26:5326–5334. https://doi.org/10.1200/JCO.2008.16.3212
Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, Steffens CC, Alonso-Orduña V, Schlichting C, Reyes-Rivera I, Bendahmane B, André T, Kubicka S, ML18147 Study Investigators (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37. https://doi.org/10.1016/S1470-2045(12)70477-1
von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, Villanueva C, Romieu G, Lang I, Ciruelos E, De Laurentiis M, Veyret C, de Ducla S, Freudensprung U, Srock S, Gligorov J (2014) Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol 15:1269–1278. https://doi.org/10.1016/S1470-2045(14)70439-5
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was not obtained, because our study was retrospective analysis.
Rights and permissions
About this article
Cite this article
Takasaki, K., Miyamoto, M., Takano, M. et al. Addition of bevacizumab to gemcitabine for platinum-resistant recurrent ovarian cancer: a retrospective analysis. Cancer Chemother Pharmacol 81, 809–814 (2018). https://doi.org/10.1007/s00280-018-3552-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3552-5