Abstract
Purpose
Ovarian cancer remains a most malignant female cancer nowadays. The acquisition of chemoresistance to common-used cisplatin-based chemotherapy results in a decreased overall patient survival. The present study is aimed to investigate the role and mechanism by which miR-139/ ATPases7A/B axis modulates the chemoresistance of ovarian cancer to cisplatin-based chemotherapy.
Methods
The expression of miR-139 in cisplatin-sensitive (n = 23) and cisplatin-resistant (n = 14) ovarian cancer tissues and cell lines (CAOV-3 and SNU119) was determined using real-time PCR assays; its effect on ovarian cancer cell chemoresistance to different concentrations of cisplatin was then assessed by measuring the cell viability using MTT assays. Next, miR-139 binding to the 3′UTR of ATP7A/B was confirmed using luciferase reporter gene assays. Finally, the combined effect of miR-139 and ATP7A/B on the chemoresistance of ovarian cancer cell was assessed.
Results
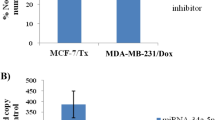
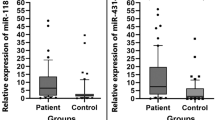
miR-139 expression was down-regulated in cisplatin-resistant ovarian cancer tissues (**P < 0.01) and reduced by cisplatin treatment in ovarian cell lines (*P < 0.05, **P < 0.01); miR-139 could enhance cisplatin-induced suppression on ovarian cancer cell viability, shown as reduced lC50 values; ATP7A and ATP7B protein levesincreased approximately 2 ~ fold-changein cisplatin-resistant cell lines. MiR-139 directly bound to the 3′UTR of ATP7A/B, respectively; miR-139 inhibition increased lC50 values whereas ATP7A/B knockdown reduced lC50 values of CAOV-3 and SNU119 cell lines under cisplatin treatment; the effect of miR-139 inhibition could be partially attenuated by ATP7A/B knockdown.
Conclusions
MiR-139/ATP7A/B axis can be a reliable biomarker for ovarian cancer diagnosis, and may affect the chemoresistance of ovarian cancer to cisplatin-based chemotherapy; rescuing miR-139 expression thus to inhibit ATP7A/B might contribute to dealing with the chemoresistance of ovarian cancer.






Similar content being viewed by others
References
Webb PM, Jordan SJ (2017) Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 41:3–14. https://doi.org/10.1016/j.bpobgyn.2016.08.006
Jayde V, White K, Blomfield P (2009) Symptoms and diagnostic delay in ovarian cancer: a summary of the literature. Contemp Nurse 34(1):55–65
Jayde V, Boughton M (2012) The diagnostic journey of ovarian cancer: a review of the literature and suggestions for practice. Contemp Nurse 41(1):5–17. https://doi.org/10.5172/conu.2012.41.1.5
Winner KR, Steinkamp MP, Lee RJ, Swat M, Muller CY, Moses ME, Jiang Y, Wilson BS (2016) Spatial modeling of drug delivery routes for treatment of disseminated ovarian cancer. Cancer Res 76(6):1320–1334. https://doi.org/10.1158/0008-5472.CAN-15-1620
Ween MP, Armstrong MA, Oehler MK, Ricciardelli C (2015) The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit Rev Oncol Hematol 96(2):220–256. https://doi.org/10.1016/j.critrevonc.2015.05.012
Ali AY, Farrand L, Kim JY, Byun S, Suh JY, Lee HJ, Tsang BK (2012) Molecular determinants of ovarian cancer chemoresistance: new insights into an old conundrum. Ann N Y Acad Sci 1271:58–67. https://doi.org/10.1111/j.1749-6632.2012.06734.x
Mihanfar A, Fattahi A, Nejabati HR (2017) MicroRNA-mediated drug resistance in ovarian cancer. J Cell Physiol. https://doi.org/10.1002/jcp.26060
van Jaarsveld MT, Helleman J, Berns EM, Wiemer EA (2010) MicroRNAs in ovarian cancer biology and therapy resistance. Int J Biochem Cell Biol 42(8):1282–1290. https://doi.org/10.1016/j.biocel.2010.01.014
Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, Johnstone CN, Chen XM, Liu CG, Huang Q, Katsaros D, Calin GA, Weber BL, Butzow R, Croce CM, Coukos G, Zhang L (2008) MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res 68(24):10307–10314. https://doi.org/10.1158/0008-5472.CAN-08-1954
Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK (2009) MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther 8(5):1055–1066. https://doi.org/10.1158/1535-7163.MCT-08-1046
Pal MK, Jaiswar SP, Dwivedi VN, Tripathi AK, Dwivedi A, Sankhwar P (2015) MicroRNA: a new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol Med 12(4):328–341. https://doi.org/10.7497/j.issn.2095-3941.2015.0024
Hu Y, Deng C, Zhang H, Zhang J, Peng B, Hu C (2017) Long non-coding RNA XIST promotes cell growth and metastasis through regulating miR-139-5p mediated Wnt/beta-catenin signaling pathway in bladder cancer. Oncotarget 8(55):94554–94568. https://doi.org/10.18632/oncotarget.21791
Huang P, Xi J, Liu S (2016) MiR-139-3p induces cell apoptosis and inhibits metastasis of cervical cancer by targeting NOB1. Biomed Pharmacother 83:850–856. https://doi.org/10.1016/j.biopha.2016.07.050
Yue S, Wang L, Zhang H, Min Y, Lou Y, Sun H, Jiang Y, Zhang W, Liang A, Guo Y, Chen P, Lv G, Wang L, Zong Q, Li Y (2015) miR-139-5p suppresses cancer cell migration and invasion through targeting ZEB1 and ZEB2 in GBM. Tumour Biol 36(9):6741–6749. https://doi.org/10.1007/s13277-015-3372-8
Zhang HD, Sun DW, Mao L, Zhang J, Jiang LH, Li J, Wu Y, Ji H, Chen W, Wang J (2015) MiR-139-5p inhibits the biological function of breast cancer cells by targeting Notch1 and mediates chemosensitivity to docetaxel. Biochem Biophys Res Commun 465(4):702–713
Liu H, Yin Y, Hu Y, Feng Y, Bian Z, Yao S, Li M, You Q, Huang Z (2016) miR-139-5p sensitizes colorectal cancer cells to 5-fluorouracil by targeting NOTCH-1. Pathol Res Pract 212(7):643–649
Ke X, Ke S, Xin L, Li Y, Nagao N, Li J, Liu J, Yin P (2016) MiR-139-5p reverses CD44+/CD133+-associated multidrug resistance by downregulating NOTCH1 in colorectal carcinoma cells. Oncotarget 7(46):75118
Li Q, Liang X, Wang Y, Meng X, Xu Y, Cai S, Wang Z, Liu J, Cai G (2016) miR-139-5p inhibits the epithelial-mesenchymal transition and enhances the chemotherapeutic sensitivity of colorectal cancer cells by downregulating BCL2. Sci Rep 6:27157
Sun S, Cai J, Yang Q, Zhao S, Wang Z (2017) The association between copper transporters and the prognosis of cancer patients undergoing chemotherapy: a meta-analysis of literatures and datasets. Oncotarget 8(9):16036–16051. https://doi.org/10.18632/oncotarget.13917
Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, Spannuth WA, Tanaka T, Shahzad MM, Lin YG, Nick AM, Danes CG, Lee JW, Jennings NB, Vivas-Mejia PE, Wolf JK, Coleman RL, Siddik ZH, Lopez-Berestein G, Lutsenko S, Sood AK (2009) Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res 15(11):3770–3780. https://doi.org/10.1158/1078-0432.CCR-08-2306
Kalayda GV, Wagner CH, Buss I, Reedijk J, Jaehde U (2008) Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer 8(1):175
Sabbatini G, Baldoni G (2004) Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol 66(1):25–32
Safaei R, Holzer AK, Katano K, Samimi G, Howell SB (2004) The role of copper transporters in the development of resistance to Pt drugs. J Inorg Biochem 98(10):1607–1613
Samimi G, Varki NM, Wilczynski S, Safaei R, Alberts DS, Howell SB (2003) Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin Cancer Res 9(16 Pt 1):5853
Nakayama K, Kanzaki A, Terada K, Mutoh M, Ogawa K, Sugiyama T, Takenoshita S, Itoh K, Yaegashi N, Miyazaki K (2004) Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin Cancer Res 10(8):2804–2811
Agarwal V, Bell GW, Nam JW, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. Elife. https://doi.org/10.7554/eLife.05005
Current FIGO. staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia (2009). Int J Gynaecol Obstet 105 (1):3–4
Scully RE, Sobin LH (1987) Histologic typing of ovarian tumors. Arch Pathol Lab Med 111(9):794–795
Shepherd JH (1989) Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol 96(8):889–892
Zhu X, Shen H, Yin X, Long L, Chen X, Feng F, Liu Y, Zhao P, Xu Y, Li M, Xu W, Li Y (2017) IL-6R/STAT3/miR-204 feedback loop contributes to cisplatin resistance of epithelial ovarian cancer cells. Oncotarget 8(24):39154–39166. https://doi.org/10.18632/oncotarget.16610
Wang Y, Kim S, Kim IM (2014) Regulation of metastasis by microRNAs in ovarian cancer. Front Oncol 4:143. https://doi.org/10.3389/fonc.2014.00143
Park YT, Jeong JY, Lee MJ, Kim KI, Kim TH, Kwon YD, Lee C, Kim OJ, An HJ (2013) MicroRNAs overexpressed in ovarian ALDH1-positive cells are associated with chemoresistance. J Ovarian Res 6(1):18. https://doi.org/10.1186/1757-2215-6-18
Cheng W, Liu T, Wan X, Gao Y, Wang H (2012) MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J 279(11):2047–2059. https://doi.org/10.1111/j.1742-4658.2012.08589.x
Wang Z, Ting Z, Li Y, Chen G, Lu Y, Hao X (2013) microRNA-199a is able to reverse cisplatin resistance in human ovarian cancer cells through the inhibition of mammalian target of rapamycin. Oncol Lett 6(3):789–794. https://doi.org/10.3892/ol.2013.1448
Chen X, Lu P, Wang DD, Yang SJ, Wu Y, Shen HY, Zhong SL, Zhao JH, Tang JH (2016) The role of miRNAs in drug resistance and prognosis of breast cancer formalin-fixed paraffin-embedded tissues. Gene 595(2):221–226. https://doi.org/10.1016/j.gene.2016.10.015
Chen Y, Gao DY, Huang L (2015) In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 81:128–141. https://doi.org/10.1016/j.addr.2014.05.009
Aigner A (2011) MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl) 89(5):445–457. https://doi.org/10.1007/s00109-010-0716-0
Dwivedi SK, Mustafi SB, Mangala LS, Jiang D, Pradeep S, Rodriguez-Aguayo C, Ling H, Ivan C, Mukherjee P, Calin GA, Lopez-Berestein G, Sood AK, Bhattacharya R (2016) Therapeutic evaluation of microRNA-15a and microRNA-16 in ovarian cancer. Oncotarget 7(12):15093–15104. https://doi.org/10.18632/oncotarget.7618
Zhao M, Su Z, Zhang S, Zhuang L, Xie Y, Li X (2016) Suppressive role of MicroRNA-148a in cell proliferation and invasion in ovarian cancer through targeting transforming growth factor-beta-induced 2. Oncol Res 24(5):353–360. https://doi.org/10.3727/096504016X14685034103275
Zhao S, Wen Z, Liu S, Liu Y, Li X, Ge Y, Li S (2015) MicroRNA-148a inhibits the proliferation and promotes the paclitaxel-induced apoptosis of ovarian cancer cells by targeting PDIA3. Mol Med Rep 12(3):3923–3929. https://doi.org/10.3892/mmr.2015.3826
Zhou X, Zhao F, Wang ZN, Song YX, Chang H, Chiang Y, Xu HM (2012) Altered expression of miR-152 and miR-148a in ovarian cancer is related to cell proliferation. Oncol Rep 27(2):447–454. https://doi.org/10.3892/or.2011.1482
Wen Z, Zhao S, Liu S, Liu Y, Li X, Li S (2015) MicroRNA-148a inhibits migration and invasion of ovarian cancer cells via targeting sphingosine-1-phosphate receptor 1. Mol Med Rep 12(3):3775–3780. https://doi.org/10.3892/mmr.2015.3827
Benson EA, Skaar TC, Liu Y, Nephew KP, Matei D (2015) Carboplatin with decitabine therapy, in recurrent platinum resistant ovarian cancer, alters circulating miRNAs concentrations: A Pilot Study. PLoS One 10(10):e0141279. https://doi.org/10.1371/journal.pone.0141279
Author information
Authors and Affiliations
Contributions
Fang Xiao designed and performed the experiments, wrote the manuscript. Fang Xiao and Yueran Li have contributed to experimental work and data analysis. Yajun Wan and Min Xue conducted the experiments and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Author Fang Xiao declares that she has no conflict of interest. Author Yueran Li declares that she has no conflict of interest. Author Yajun Wan declares that she has no conflict of interest. Author Min Xue declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
280_2018_3548_MOESM1_ESM.tif
Fig. S1 Transfection efficiency and expression level confirmation (A-B) miR-139 mimics was transfected into CAOV3 and SNU119 cells to achieve ectopic miR-139 expression, as verified using real-time PCR assays. (C) The expression of ATP7A in CAOV3, SNU119, CAOV3/cDDP and SNU119/cDDP cells was determined by Immunoblotting. (D) The expression of miR-139 in CAOV3, SNU119, CAOV3/cDDP and SNU119/cDDP cells was determined by RT-PCR. (E) CAOV3 and SNU119 cells were transfected with miR-139 inhibitor to achieve miR-139 inhibition, as verified using real-time PCR assays. (F) The expression of ATP7B in CAOV3, SNU119, CAOV3/cDDP and SNU119/cDDP cells was determined by Immunoblotting. (TIF 773 KB)
Rights and permissions
About this article
Cite this article
Xiao, F., Li, Y., Wan, Y. et al. MircroRNA-139 sensitizes ovarian cancer cell to cisplatin-based chemotherapy through regulation of ATP7A/B. Cancer Chemother Pharmacol 81, 935–947 (2018). https://doi.org/10.1007/s00280-018-3548-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3548-1