Abstract
Purpose
Low skeletal muscle mass (sarcopenia) has been reported to have an influence on survival and toxicities of chemotherapy in several solid tumors. The impact of sarcopenia on the treatment of ovarian cancers has not been determined. The present study aimed to evaluate correlation between sarcopenia and toxicities of chemotherapy in ovarian cancers.
Methods
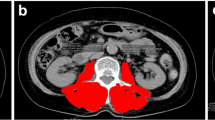
Medical charts of the ovarian cancer patients that received chemotherapy with paclitaxel and carboplatin at our hospital between 2010 and 2015 were retrospectively reviewed. Muscle areas of bilateral psoas major muscles at the fifth lumbar vertebra were measured using images obtained by computed tomography. The volume of muscle and clinicopathological factors were evaluated for toxicities of chemotherapy. The protocol of the present study was approved by the institutional review board of our institution.
Results
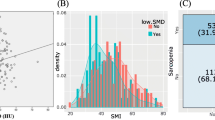
A total of 76 patients with ovarian cancers were identified, and enrolled in the present study. Median psoas index (PI, the psoas muscle major cross-sectional area divided by the height squared) was 583 mm2/m2 (range 326–999). The patients with low PI developed peripheral neuropathy more frequently compared with those with high PI (32 vs. 11%; P = 0.047). PI value was not associated with other toxicities such as neutropenia and thrombocytopenia. PI value was associated with grade 2 or higher peripheral neuropathy in univariate analysis (OR = 3.92; 95% CI 1.21–15.32; P = 0.02) and multivariate analysis (OR = 3.93; 95% CI 1.17–15.87; P = 0.03).
Conclusions
PI value was significantly associated with peripheral neuropathy induced by combination therapy with paclitaxel and carboplatin in ovarian cancer patients. Although further studies are needed to confirm the results, the volume of skeletal muscle mass could be a potential biomarker to predict toxicities in ovarian cancer patients.


Similar content being viewed by others
References
Solheim TS, Fayers PM, Fladvad T, Tan B, Skorpen F, Fearon K, Baracos VE, Klepstad P, Strasser F, Kaasa S, European Palliative Care Research Collaborative (EPCRC) and the European Pharmacogenetic Study (EPOS) (2012) Is there a genetic cause of appetite loss?-an explorative study in 1853 cancer patients. J Cachexia Sarcopenia Muscle 3(3):191–198
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 57:58–67
Sato T, Aoyama T, Hayashi T, Segami K, Kawabe T, Fujikawa H, Yamada T, Yamamoto N, Oshima T, Rino Y, Masuda M, Ogata T, Cho H, Yoshikawa T (2016) Impact of preoperative hand grip strength on morbidity following gastric cancer surgery. Gastric Cancer 19(3):1008–1015
Chemama S, Bayar MA, Lanoy E, Ammari S, Stoclin A, Goéré D, Elias D, Raynard B, Antoun S (2016) Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann Surg Oncol 23(12):3891–3898
Jung HW, Kim JW, Kim JY, Kim SW, Yang HK, Lee JW, Lee KW, Kim DW, Kang SB, Kim KI, Kim CH, Kim JH (2015) Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer 23(3):687–694
Rutten IJ, van Dijk DP, Kruitwagen RF, Beets-Tan RG, Olde Damink SW, van Gorp T (2016) Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle 7(4):458–466
Kumar A, Moynagh MR, Multinu F, Cliby WA, McGree ME, Weaver AL, Young PM, Bakkum-Gamez JN, Langstraat CL, Dowdy SC, Jatoi A, Mariani A (2016) Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecol Oncol 142(2):311–316
Brewer JR, Morrison G, Dolan ME, Fleming GF (2016) Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol 140(1):176–183
Bolis G, Scarfone G, Raspagliesi F, Mangili G, Danese S, Scollo P, Lo Russo D, Villa A, Aimone PD, Scambia G (2010) Paclitaxel/carboplatin versus topotecan/paclitaxel/carboplatin in patients with FIGO suboptimally resected stage III-IV epithelial ovarian cancer a multicenter, randomized study. Eur J Cancer 46(16):2905–2912
Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, Bologna A, Weber B, Raspagliesi F, Panici PB, Cormio G, Sorio R, Cavazzini MG, Ferrandina G, Breda E, Murgia V, Sacco C, Cinieri S, Salutari V, Ricci C, Pisano C, Greggi S, Lauria R, Lorusso D, Marchetti C, Selvaggi L, Signoriello S, Piccirillo MC, Di Maio M, Perrone F, Multicentre Italian Trials in Ovarian cancer (MITO-7).; Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens et du sein (GINECO).; Mario Negri Gynecologic Oncology (MaNGO).; European Network of Gynaecological Oncological Trial Groups (ENGOT-OV-10).; Gynecologic Cancer InterGroup (GCIG) Investigators (2014) Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 15(4):396–405
Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K, Japanese Gynecologic Oncology Group (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374(9698):1331–1338
Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, Parkin D, Paul J, Hay A, Kaye SB, Scottish Gynaecological Cancer Trials Group (2004) Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst 96(22):1682–1691
Meriggioli MN, Roubenoff R (2015) Prospect for pharmacological therapies to treat skeletal muscle dysfunction. Calcif Tissue Int 96(3):234–242
Mithal A, Bonjour JP, Boonen S, Burckhardt P, Degens H, El Hajj Fuleihan G, Josse R, Lips P, Morales Torres J, Rizzoli R, Yoshimura N, Wahl DA, Cooper C, Dawson-Hughes B, IOF CSA Nutrition Working Group (2013) Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int 24(5):1555–1566
Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, Sawyer MB (2012) Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer 106(10):1583–1586
Acknowledgements
The authors thank MS. Hiromi Kubota for continuous contribution to our clinical study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study protocol was approved by the institutional review board of National Defense Medical College.
Rights and permissions
About this article
Cite this article
Yoshikawa, T., Takano, M., Miyamoto, M. et al. Psoas muscle volume as a predictor of peripheral neurotoxicity induced by primary chemotherapy in ovarian cancers. Cancer Chemother Pharmacol 80, 555–561 (2017). https://doi.org/10.1007/s00280-017-3395-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3395-5