Abstract
Purpose
Alectinib, a central nervous system (CNS)-active ALK inhibitor, has demonstrated efficacy and safety in ALK+ non-small-cell lung cancer that has progressed following crizotinib treatment. Other ALK inhibitors have shown concentration-dependent QTc prolongation and treatment-related bradycardia. Therefore, this analysis evaluated alectinib safety in terms of electrophysiologic parameters.
Methods
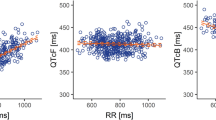
Intensive triplicate centrally read electrocardiogram (ECG) and matched pharmacokinetic data were collected across two alectinib single-arm trials. Analysis of QTcF included central tendency analysis [mean changes from baseline with one-sided upper 95% confidence intervals (CIs)], categorical analyses, and relationship between change in QTcF and alectinib plasma concentrations. Alectinib effects on other ECG parameters (heart rate, PR interval and QRS duration) were also evaluated.
Results
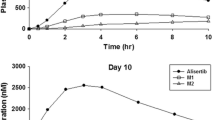
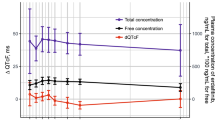
Alectinib did not cause a clinically relevant change in QTcF. The maximum mean QTcF change from baseline was 5.3 ms observed pre-dose at week 2. The upper one-sided 95% CI was <10 ms at all time points. There was no relevant relationship between change in QTcF and alectinib plasma concentrations. Alectinib treatment resulted in a generally asymptomatic exposure-dependent decrease in mean heart rate of ~11 to 13 beats per minute at week 2. No clinically relevant effects were seen on other ECG parameters. Approximately 5% of patients reported cardiac adverse events of bradycardia or sinus bradycardia; however, these were all grade 1–2.
Conclusions
Alectinib does not prolong the QTc interval or cause changes in cardiac function to a clinically relevant extent, with the exception of a decrease in heart rate which was generally asymptomatic.




Similar content being viewed by others
References
Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F (2014) ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev 40:300–306
Shaw AT, Solomon B (2011) Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res 17:2081–2086
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F, PROFILE (1014) Investigators (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–2177
Peters S (2016) Emerging options after progression during crizotinib therapy. J Clin Oncol 34:643–645
Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, Chao BH, Borghaei H, Gold KA, Zeaiter A, Bordogna W, Balas B, Puig O, Henschel V, Ou SH, study investigators (2016) Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 17:234–242
Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, Lena H, Moro-Sibilot D, Bearz A, Ramirez SV, Mekhail T, Spira A, Bordogna W, Balas B, Morcos PN, Monnet A, Zeaiter A, Kim DW (2016) Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol 34:661–668
International Conference on Harmonization (2005) Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs
Van Noord C, Eijgelsheim M, Stricker BHC (2010) Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol 70:16–23
US Food and Drug Administration (2016) Clinical pharmacology and biopharmaceutics review(s) of alectinib. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208434Orig1s000PharmR.pdf. Accessed 6 July 2016
Hsu JC, Carnac R, Henschel V, Bogman K, Martin-Facklam M, Guerini E, Balas B, Hassan Zeaiter A, Phipps A, Morcos PN, Frey N (2016) Population pharmacokinetics (popPK) and exposure-response (ER) analyses to confirm alectinib 600 mg BID dose selection in a crizotinib-progressed or intolerant population. J Clin Oncol 34(Suppl; abstr):e20598
Fridericia LS (2003) The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Ann Noninvasive Electrocardiol 8:343–351
Heinig K, Miya K, Kamei T, Guerini E, Fraier D, Yu L, Bansal S, Morcos PN (2016) Bioanalysis of alectinib and metabolite M4 in human plasma, cross-validation and impact on PK assessment. Bioanalysis 8:1465–1479
Morcos PN, Cleary Y, Guerini E, Dall G, Bogman K, De Petris L, Viteri S, Bordogna W, Yu L, Martin-Facklam M, Phipps A (2016) Clinical drug–drug interactions (DDIs) through cytochrome P450 3 A (CYP3A) for alectinib, a highly selective ALK inhibitor. Clin Pharmacol Ther 99(abstr PI-119):S62
Gadgeel S, Gandhi L, Riely G, Chiappori AA, West HL, Azada MC, Morcos PN, Lee RM, Garcia L, Yu L, Boisserie F, Di Laurenzio L, Golding S, Sato J, Yokoyama S, Tanaka T, Ou SH (2014) Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 15:1119–1128
Srinivasamaharaj S, Salame B, Rios-Perez J, Kloecker G, Perez CA (2016) The role of alectinib in the treatment of advanced ALK-rearranged non-small-cell lung cancer. Expert Rev Anticancer Ther 16:1227–1233
US Food and Drug Administration (2016) Xalkori prescribing information. http://labeling.pfizer.com/showlabeling.aspx?id=676. Accessed 26 July 2016
US Food and Drug Administration (2015) Zykadia prescribing information. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/zykadia.pdf. Accessed 26 July 2016
Ou SH, Tong WP, Azada M, Siwak-Tapp C, Dy J, Stiber JA (2013) Heart rate decrease during crizotinib treatment and potential correlation to clinical response. Cancer 119:1969–1975
Ou SH, Azada M, Dy J, Stiber JA (2011) Asymptomatic profound sinus bradycardia (heart rate 45) in non-small cell lung cancer patients treated with crizotinib. J Thorac Oncol 6:2135–2137
US Food and Drug Administration (2014) Clinical/statistical review of ceritinib. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755Orig1s000MedR.pdf. Accessed 26 Aug 2016
Acknowledgements
Third-party medical writing assistance, under the direction of the authors, was provided by Joanna Musgrove, MRes of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are employees of F. Hoffmann-La Roche Ltd. and have stock in F. Hoffmann-La Roche Ltd.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morcos, P.N., Bogman, K., Hubeaux, S. et al. Effect of alectinib on cardiac electrophysiology: results from intensive electrocardiogram monitoring from the pivotal phase II NP28761 and NP28673 studies. Cancer Chemother Pharmacol 79, 559–568 (2017). https://doi.org/10.1007/s00280-017-3253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3253-5