Abstract
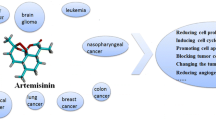
Since the late 1990s, there has been rapid multiplication of data on the anti-cancer properties of artemisinins. This article reviews the status of progress of artemisinin and its derivatives as anti-cancer agents in clinical trials, case reports, and in vitro/in vivo studies. Particular attention is laid on the combinations of artemisinins and synthetic chemodrugs to enhance the latter’s efficacy. An attempt is here made to rationalize the synergistic effects of a few common anti-cancer drugs of the anthracycline, taxane, anti-metabolite, and platinum-based drug families. The various pathways that mediate the action of artemisinins as reported over the past decade are here summarized highlighting also the biomarkers that could be used to better predict the efficacy of the sesquiterpenoids. Their main action seems to be directed toward stalling tumor cell proliferation through cell cycle arrest mediated by reactive oxygen species (ROS). The emergence of artemisinins’ nano-based formulations in combination with chemodrugs to enhance drug bioavailability and targeting as well as immunotherapy is also reviewed. The enhanced efficacy of artemisinin dimers compared to the parent molecules and standard chemotherapy is analyzed. While these therapies hold promises, it may be premature to conclude on their efficacy in the absence of clinical studies.




Similar content being viewed by others
References
Tu Y (2011) The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med 17(10):1217–1220
Lai H, Singh NP (1995) Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett 91:41–46
Moore JC, Lai H, Li J, McDougall JA, Singh NP, Chou CK (1995) Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett 98:83–87
Singh NP, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci 70:49–56
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18:767–773
Singh NP, Lai H (2004) Artemisinin Induces Apoptosis in Human Cancer Cells. Anticancer Res 24:2277–2280
Das AK (2015) Anticancer effect of antimalarial artemisinin compounds. Ann Med Health Sci Res 5(2):93–102. doi:10.4103/2141-9248.153609
Crespo-Ortiz MP, Wei MQ (2012) Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol. doi:10.1155/2012/247597
Krishna S, Ganapathi S, Ster IC, Saeed MEM, Cowand M, Finlayson C et al (2015) A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine 2:82–90
Deliu IC, Ciurea P, Neagoe D, Bezna MC, Gheonea IA et al (2015) Evaluation of angiogenesis in colorectal cancer. Curr Health Sci J 41(2):145–151
Jansen FH, Adoubi I, J C KC, DE Cnodder T, Jansen N, Tschulakow A, Efferth T (2011) First study of oral Artenimol-R in advanced cervical cancer: clinical benefit, tolerability and tumor markers. Anticancer Res 31(12):4417–4422
Zhang ZY, Yu SQ, Miao LY, Huang XY, Zhang XP, Zhu YP et al (2008) Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: A randomized controlled trial. J Chin Integr Med 6(2):134–138
Ericsson T, Blank A, von Hagens C, Ashton M, Äbelö A (2014) Population pharmacokinetics of artesunate and dihydroartemisinin during long-term oral administration of artesunate to patients with metastatic breast cancer. Eur J Clin Pharmacol 70(12):1453–1463. doi:10.1007/s00228-014-1754-2
Genovese RF, Newman DB, Brewer TG (2000) Behavioral and neural toxicity of the artemisinin antimalarial, arteether, but not artesunate and artelinate, in rats. Pharmacol Biochem Behav 67(1):37–44
König M, von Hagens C, Hoth S, Baumann I, Walter-Sack I, Edler L et al (2016) Investigation of ototoxicity of artesunate as add-on therapy in patients with metastatic or locally advanced breast cancer: new audiological results from a prospective, open, uncontrolled, monocentric phase I study. Cancer Chemother Pharmacol 77(2):413–427. doi:10.1007/s00280-016-2960-7
Michaelsen F-WS, Saeed MM, Schwarzkopf J, Efferth T (2015) Activity of Artemisia annua and artemisinin derivatives in prostate carcinoma. Phytomedicine 22:1223–1231
Singh NP, Verma KB (2002) Case report of a laryngeal squamous cell carcinoma treated with artesunate. Arch. Oncol 10(4):279–280
Singh NP, Panwar VK (2006) Case Report of a Pituitary Macroadenoma Treated With Artemether. Integr Cancer Ther 5(4):391–394
Rowen RJ (2002) Artemisinin: from Malaria to cancer treatment. Townsend Letter for Doctors & Patients pp 86–88
Berger TG, Dieckmann D, Efferth T, Schultz ES, Funk JO, Baur A et al (2005) Artesunate in the treatment of metastatic uveal melanoma–first experiences. Oncol Rep 14(6):1599–1603
The Cancer Cure Foundation. http://www.cancure.org/12-links-page/43-artemesia. Accessed 11 Oct 2016
Uhl M, Schwab S, Efferth T (2016) Fatal liver and bone marrow toxicity by combination treatment of dichloroacetate and artesunate in a glioblastoma multiforme patient: case report and review of the literature. Front Oncol 6:204–209. doi:10.3389/fonc.2016.00204
Reungpatthanaphong P, Mankhetkorn S (2002) Modulation of multidrug resistance by artemisinin, artesunate and dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines. Biol Pharm Bull 25(12):1555–1561
Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M et al (2007) Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One 2(8):e693. doi:10.1371/journal.pone.0000693
Wu G-S, Lu J-J, Guo J-J, Huang M-Q, Gan L, Chen X-P et al (2013) Synergistic anti-cancer activity of the combination of dihydroartemisinin and doxorubicin in breast cancer cells. Pharmacol Rep 65:453–459
Eckstein-Ludwig U, Webb RJ, van Goethem IDA, East JM, Lee AG, Kimura M et al (2003) Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957–961
Riganti C, Doublier S, Viarisio D, Miraglia E, Pescarmona G, Ghigo D et al (2009) Artemisinin induces doxorubicin resistance in human colon cancer cells via calcium-dependent activation of HIF-1a and P-glycoprotein overexpression. Br J Pharmacol 156:1054–1066
Lucibello M, Gambacurta A, Zonfrillo M, Pierimarchi P, Serafino A, Rasi G et al (2011) TCTP is a critical survival factor that protects cancer cells from oxidative stress-induced cell-death. Exp Cell Res 317:2479–2489
Lucibello M, Adanti S, Antelmi E, Dezi D, Ciafrè S, Carcangiu ML et al (2015) Phospho-TCTP as a therapeutic target of dihydroartemisinin for aggressive breast cancer cells. Oncotarget 6(7):5275–5291
Wang SJ, Gao Y, Chen H, Kong R, Jiang HC, Pan SH et al (2010) Dihydroartemisinin inactivates NF-κB and potentiates the anti-tumor effect of gemcitabine on pancreatic cancer both In vitro and In vivo. Cancer Lett 293(1):99–108
Hou J, Wang D, Zhang R, Wang H (2008) Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res 14:5519–5530
Zhao C, Gao W, Chen T (2014) Synergistic induction of apoptosis in A549 cells by dihydroartemisinin and gemcitabine. Apoptosis 19(4):668–681
Zhao C, Qin G, Gao W, Chen J, Liu H, Xi G et al (2014) Potent proapoptotic actions of dihydroartemisinin in gemcitabine-resistant A549 cells. Cell Signal 26(10):2223–2233. doi:10.1016/j.cellsig.2014.07.001
Gravett AM, Liu WM, Krishna S, Chan W-C, Haynes RK, Wilson NL et al (2010) In vitro study of the anti-cancer effects of artemisone alone or in combination with other chemotherapeutic agents. Cancer Chemother Pharmacol 67(3):569–577
van Huijsduijnen RH, Guy RK, Chibale K, Haynes RK, Peitz I, Kelter G et al (2013) Anticancer Properties of Distinct Antimalarial Drug Classes. PLoS One 8(12):e82962. doi:10.1371/journal.pone.0082962
Tan X, Chen YI, Chin B, Bieber M, Teng N et al (2014) Artemisinin derivatives synergize with paclitaxel by targeting foxm1 through raf/mek/mapk signaling pathway in ovarian cancer. Abstract 0258, 15th Biennial Meeting of the International Gynecologic Cancer Society, 8–11 November 2014, Australia
Ma RY, Tong TH, Cheung AM, Tsang AC, Leung WY et al (2005) Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J Cell Sci 118(Pt 4):795–806
Weaver BA (2014) How Taxol/paclitaxel kills cancer cells. Mol Biol Cell 25(18):2677–2681. doi:10.1091/mbc.E14-04-0916
Wu M-X (2016) Effect of artemisinin combined with cisplatin intervention on epithelial-mesenchymal transition, angiogenesis and ATP generation in MGC-803 gastric cancer cell lines. J Hainan Med Univer 22(18) (Abstract only available, article in Chinese)
Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang X et al (2015) Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol Ther 16(10):1548–1556. doi:10.1080/15384047.2015.1071738
Feng X, Li L, Jiang H, Jiang K, Jin Y et al (2014) Dihydroartemisinin potentiates the anticancer effect of cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer cells: Involvement of apoptosis and autophagy. Biochem Biophys Res Commun 444(3):376–381. doi:10.1016/j.bbrc.2014.01.053
Chen H-H, Zhou H-J, Wang W-Q, Wu G-D (2004) Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemother Pharmacol 53:423–432
Zhou HJ, Zhang JL, Li A, Wang Z, Lou XE (2010) Dihydroartemisinin improves the efficiency of chemotherapeutics in lung carcinomas In vivo and inhibits murine Lewis lung carcinoma cell line growth In vitro. Cancer Chemother Pharmacol 66(1):21–29
O’Neill PM, Barton VE, Ward SA (2010) The Molecular Mechanism of Action of Artemisinin—the Debate Continues. Molecules 15:1705–1721. doi:10.3390/molecules15031705
Efferth T (2015) Artemisinin–second career as anticancer drug? World J Tradit Chin Med 1(4):2–25
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al (2016) Ferroptosis: process and function. Cell Death Differ 23:369–379
Li Q, Weina P, Hickman M (2013) The use of artemisinin compounds as angiogenesis inhibitors to treat cancer, Chap. 7, 10.5772/54109
Dong F, Tian H, Yan S, Li L, Dong X et al (2015) Dihydroartemisinin inhibits endothelial cell proliferation through the suppression of the ERK signaling pathway. Int J Mol Med 35(5):1381–1387. doi:10.3892/ijmm.2015.2140
Zhou Y, Li W, Xiao Y (2016) Profiling of Multiple Targets of Artemisinin Activated by Hemin in Cancer Cell Proteome. ACS Chem Biol. doi:10.1021/acschembio.5b01043
Tran KQ, Tin AS, Firestone GL (2014) Artemisinin triggers a G1 cell cycle arrest of human Ishikawa endometrial cancer cells and inhibits cyclin-dependent kinase-4 promoter activity and expression by disrupting nuclear factor-κB transcriptional signaling. Anticancer Drugs 25(3):270–281. doi:10.1097/CAD.0000000000000054
Tin AS, Sundar SN, Tran KQ, Park AH, Poindexter KM, Firestone GL (2012) Antiproliferative effects of artemisinin on human breast cancer cells requires the downregulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anticancer Drugs 23(4):370–379. doi:10.1097/CAD.0b013e32834f6ea8
Willoughby JA Sr, Sundar SN, Cheung M, Tin AS, Mondiano J, Firestone GL (2009) Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the Cyclin-dependent Kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem 284(4):2203–2213. doi:10.1074/jbc.M804491200
Zhao Y, Jiang W, Li B, Yao Q, Dong J, Cen Y et al (2011) Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int Immunopharmacol 11(12):2039–2046. doi:10.1016/j.intimp.2011.08.017
Chen K, Shou LM, Lin F, Duan WM, Wu MY, Xie X et al (2014) Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anticancer Drugs 25(6):652–662. doi:10.1097/CAD.0000000000000089
Jiang Z, Chai J, Chuang HH, Li S, Wang T, Cheng Y et al (2012) Artesunate induces G0/G1 cell cycle arrest and iron-mediated mitochondrial apoptosis in A431 human epidermoid carcinoma cells. Anticancer Drugs 23(6):606–613. doi:10.1097/CAD.0b013e328350e8ac
Huang Z, Huang X, Jiang D, Zhang Y, Huang B, Luo G (2016) Dihydroartemisinin inhibits cell proliferation by induced G1 arrest and apoptosis in human nasopharyngealcarcinoma cells. J Can Res Ther 12(1):244–247
Sun H, Meng X, Han J, Zhang Z, Wang B et al (2013) Anti-cancer activity of DHA on gastric cancer–an in vitro and in vivo study. Tumour Biol 34(6):3791–3800. doi:10.1007/s13277-013-0963-0
Chen H, Sun B, Wang S, Pan S, Gao Y, Bai X et al (2010) Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-κB. J Cancer Res Clin Oncol 136(6):897–903
D’Alessandro S, Basilico N, Corbett Y, Scaccabarozzi D, Omodeo-Salè F et al (2011) Hypoxia modulates the effect of dihydroartemisinin on endothelial cells. Biochem Pharmacol 82(5):476–484. doi:10.1016/j.bcp.2011.06.002
Wartenberg M, Wolf S, Budde P, Grünheck F, Acker H, Hescheler J et al (2003) The Antimalaria Agent Artemisinin Exerts Antiangiogenic Effects in Mouse Embryonic Stem Cell-Derived Embryoid Bodies. Lab Invest 83(11):1647–1655
Jia J, Qin Y, Zhang L, Guo C, Wang Y et al (2016) Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol Med Rep 13(5):4461–4468. doi:10.3892/mmr.2016.5073
Tong Y, Liu Y, Zheng H, Zheng L, Liu W et al (2016) Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget 7(21):31413–31428
Eling N, Lukas R, Hazin J, Hamacher-Brady A, Brady NR (2015) Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2:517–532
Button RW, Lin F, Ercolano E, Vincent JH, Hu B, Hanemann CO et al (2014) Artesunate induces necrotic cell death in schwannoma cells. Cell Death Dis 5:e1466. doi:10.1038/cddis.2014.434
Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N et al (2011) Artesunate activates mitochondrial apoptosis in breast cancer cells via iron catalyzed lysosomal reactive oxygen species production. J Biol Chem 286(8):6587–6601
Steinbrück L, Pereira G, Efferth T (2010) Effects of artesunate on cytokinesis and G2/M cell cycle progression of tumour cells and budding yeast. Cancer Genom Proteom 7(6):337–346
Jeong DE, Song HJ, Lim S, Jeong Lee S, Lim JE et al (2015) Repurposing the anti-malarial drug artesunate as a novel therapeutic agent for metastatic renal cell carcinoma due to its attenuation of tumor growth, metastasis, and angiogenesis. Oncotarget 6(32):33046–33064
Greenshields AL, Shepherd TG, Hoskin DW (2016) Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol Carcinog. doi:10.1002/mc.22474
Jiao Y, Ge C-M, Meng Q-H, Cao J-P, Tong J, Fan S-J (2007) Dihydroartemisinin is an inhibitor of ovarian cancer cell growth. Acta Pharmacol Sin 28(7):1045–1056
Wang Z, Hu W, Zhang J-L, Wu X-H, Zhou H-J (2012) Dihydroartemisinin induces autophagy and inhibits the growth of iron-loaded human myeloid leukemia K562 cells via ROS toxicity. FEBS Open Bio 2:103–112. doi:10.1016/j.fob.2012.05.002
Du XX, Li YJ, Wu CL, Zhou JH, Han Y et al (2013) Initiation of apoptosis, cell cycle arrest and autophagy of esophageal cancer cells by dihydroartemisinin. Biomed Pharmacother 67(5):417–424. doi:10.1016/j.biopha.2013.01.013
Lin R, Zhang Z, Chen L, Zhou Y, Zou P et al (2016) Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett 381(1):165–175. doi:10.1016/j.canlet.2016.07.033
Hui HY, Wu N, Wu M, Liu Y, Xiao SX et al Zhang MF (2016) Dihydroartemisinin suppresses growth of squamous cell carcinoma A431 cells by targeting the Wnt/β-catenin pathway. Anticancer Drugs 27(2):99–105. doi:10.1097/CAD.0000000000000307
Kim SH, Kang SH, Kang BS (2016) Therapeutic effects of dihydroartemisinin and transferrin against glioblastoma. Nutr Res Pract 10(4):393–397
Yang N-D, Tan S-H, Ng S, Shi Y, Zhou J et al (2014) Artesunate Induces Cell Death in Human Cancer Cells via Enhancing Lysosomal Function and Lysosomal Degradation of Ferritin. J Biol Chem 289(48):33425–33441. doi:10.1074/jbc.M114.564567
Mercer AE, Copple IM, Maggs JL, O’Neill PM, Park BK (2011) The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J Biol Chem 286(2):987–996. doi:10.1074/jbc.M110.144188
Du, JH., Zhang, HD., Ma, ZJ., Ji, KM. (2010) Artesunate induces oncosis-like cell death In vitro and has antitumor activity against pancreatic cancer xenografts In vivo. Cancer Chemother Pharma 65:895–902
Liou G-Y, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44(5). doi:10.3109/10715761003667554
Schieber M, Chandel NS (2014) ROS Function in Redox Signaling and Oxidative Stress. Curr Biol 24:R453–R462. doi:10.1016/j.cub.2014.03.034
Poillet-Perez L, Despouy G, Delage-Mourroux R, Boyer-Guittaut M (2015) Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol 4:184–192
Firestone GL, Sundar SN (2009) Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med 11:e32. doi:10.1017/S1462399409001239
Huang C, Ba Q, Yue Q, Li J, Li J, Chu R, Wang H (2013) Artemisinin rewires the protein interaction network in cancer cells: network analysis, pathway identification, and target prediction. Mol BioSyst 9:3091–3100. doi:10.1039/C3MB70342H
He Q, Shi J, Shen XL, An J, Sun H, Wang L et al (2010) Dihydroartemisinin upregulates death receptor 5 expression and cooperates with TRAIL to induce apoptosis in human prostate cancer cells. Cancer Biol Ther 9(10):819–824
Konkimalla VB, Blunder M, Korn B, Soomro SA, Jansen H, Chang W et al (2008) Effect of artemisinins and other endoperoxides on nitric oxide-related signaling pathway in RAW 264.7 mouse macrophage cells. Nitric Oxide 19(2):184–191. doi:10.1016/j.niox.2008.04.008
Lai HC, Singh NP, Sasaki T (2013) Development of artemisinin compounds for cancer treatment. Invest New Drugs 31(1):230–246. doi:10.1007/s10637-012-9873-z
Lai H, Nakase I, Lacoste E, Singh NP, Sasaki T (2009) Artemisinin-transferrin conjugate retards growth of breast tumors in the rat. Anticancer Res 29:3807–3810
Bhadra D, Bhadra S, Jain NK (2005) Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J Pharm Pharmaceut Sci 8(3):467–482
Chen Y, Lin X, Park H, Greever R (2009) Study of artemisinin nanocapsules as anticancer drug delivery systems. Nanomedicine 5(3):316–322. doi:10.1016/j.nano.2008.12.005
Letchmanan K, Shen S-C, Kiong Ng W, Tan RBH (2015) Enhanced dissolution and stability of artemisinin by nano-confinement in ordered mesoporous SBA-15 particles. Microencapsul 32(4):390–400. doi:10.3109/02652048.2015.1035684
Dai L, Wang L, Deng L, Liu J, Lei J, Li D, He J (2014) Novel multiarm polyethylene glycol-dihydroartemisinin conjugates enhancing therapeutic efficacy in non-small-cell lung Cancer. Sci Rep 4:5871. doi:10.1038/srep05871
Lu W-F, Chen S-F, Wen Z-Y, Li Q, Chen J-H (2012) In vitro evaluation of efficacy of dihydroartemisinin-loaded methoxy poly(ethylene glycol)/poly(L-lactic acid) amphiphilic block copolymeric micelles. J Appl Polym Sci. doi:10.1002/APP.38518
Righeschi C, Coronnello M, Mastrantoni A, Isacchi B, Bergonzi MC et al (2014) Strategy to provide a useful solution to effective delivery of dihydroartemisinin: Development, characterization and in vitro studies of liposomal formulations. Colloids Surf B Biointerfaces 116:121–127
Dadgar N, Esfahani MKM, Torabi S, Alavi SE, Akbarzadeh A (2013) Effects of nanoliposomal and pegylated nanoliposomal artemisinin in treatment of breast cancer. Ind J Clin Biochem. doi:10.1007/s12291-013-0389-x
Sun Q, Teong B, Chen I-F, Chang SJ, Gao J, Kuo S-M (2014) Enhanced apoptotic effects of dihydroartemisinin-aggregated gelatin and hyaluronan nanoparticles on human lung cancer cells. J Biomed Mater Res Part B 102B:455–462
Wang Z, Yu Y, Ma J, Zhang H, Zhang H, Wang X et al (2012) LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol Pharm 9:2646–2657. doi:10.1021/mp3002107
Dwivedi A, Mazumder A, du Plessis L, du Preez JL, Haynes RK, du Plessis J (2015) In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomedicine 11(8):2041–2050. doi:10.1016/j.nano.2015.07.010
Liu K, Dai L, Li C, Liu J, Wang L, Lei J (2016) Self-assembled targeted nanoparticles based on transferrin modified eight-arm-polyethylene glycol–dihydroartemisinin conjugate. Sci Rep 6:29461. doi:10.1038/srep29461
Fu J, Zhu Y (2017) Lysosomes activating chain reactions against cancer cells with a pH-switched prodrug/procatalyst co-delivery nanosystem. J Mater Chem B 7(5):996–1004. doi:10.1039/C6TB02820A
Ma W, Xu A, Ying J, Li B, Jin Y (2015) Biodegradable core–shell copolymer-phospholipid nanoparticles for combination chemotherapy: an in vitro study. J Biomed Nanotechnol 11:1193–1200
Li X-Y, Zhao Y, Sun M-G, Shi J-F, Ju R-J, Zhang C-X et al (2014) Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials 35:5591–5604
Fröhlich T, Karagöz AC, Reiter C, Tsogoeva SB (2016) Artemisinin-derived dimers: potent antimalarial and anti-cancer agents. J Med Chem. doi:10.1021/acs.jmedchem.5b01380
Alagbala AA, McRiner AJ, Borstnik K, Labonte T, Chang W et al (2006) Biological mechanisms of action of novel C-10 non-acetal trioxane dimers in prostate cancer cell lines. J Med Chem 49:7836–7842
Stockwin LH, Han B, Yu SX, Hollingshead MG, Elsohly MA et al (2009) Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction. Int J Cancer 125:1266–1275
Posner GH, McRiner AJ, Paik IH, Sur S, Borstnik K et al (2004) Anticancer and antimalarial efficacy and safety of artemisinin-derived trioxane dimers in rodents. J Med Chem 47:1299–1301
Lombard MC, N’Da DD, Breytenbach JC, Kolesnikova NI, Tran Van Ba C, Wein S, Norman J, Denti P, Vial H, Wiesner L (2012) Antimalarial and anticancer activities of artemisinin–quinoline hybrid-dimers and pharmacokinetic properties in mice. Eur J Pharm Sci 47:834–841
Singh NP, Lai HC, Park JS, Gerhardt TE, Kim BJ, Wang S, Sasaki T (2011) Effects of artemisinin dimers on rat breast cancer cells in vitro and in vivo. Anticancer Res 31:4111–4114
Fox JM, Moynihan JR, Mott BT, Mazzone JR, Anders NM et al (2016) Artemisinin-derived dimer ART-838 potently inhibited human acute leukemias, persisted in vivo, and synergized with antileukemic drugs. Oncotarget 7(6):7268–7279
Beekman AC, Barentsen ARW, Woerdenbag HJ, Van Uden W, Pras N, Konings AWT, El-Feraly FS, Galal AM, Wikstrom HV (1997) Stereochemistry-dependent cytotoxicity of some artemisinin derivatives. J Nat Prod 60:325–330
Posner GH, Paik I-H, Sur S, McRiner AJ, Borstnik K, Xie S, Shapiro TA (2003) Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high stability and efficacy. J Med Chem 46:1060–1065
Emens LA, Middleton G (2015) The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3(5):436–443. doi:10.1158/2326-6066.CIR-15-0064
Yao W, Wang F, Wang H (2016) Immunomodulation of artemisinin and its derivatives. Sci Bull. doi:10.1007/s11434-016-1105-z
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. doi:10.1038/nature01322
Wang X, Lin Y (2008) Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin 29(11):1275–1288
Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16:431–446
Hunt S, Yoshida M, Davis CEJ, Greenhill NS, Davis PF (2015) An extract of the medicinal plant Artemisia annua modulates production of inflammatory markers in activated neutrophils. J Inflamm Res 8:9–14
Williams CB, Yeh ES, Soloff AC (2016) Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2:15025–15046. doi:10.1038/npjbcancer.2015.25
Li B, Zhang R, Li J et al (2008) Antimalarial artesunate protects sepsis model mice against heat-killed Escherichia coli challenge by decreasing TLR4, TLR9 mRNA expressions and transcription factor NF-kappa B activation. Int Immunopharmacol 8:379–389
Wang Y, Huang ZQ, Wang CQ et al (2011) Artemisinin inhibits extracellular matrix metalloproteinase inducer (EMMPRIN) and matrix metalloproteinase-9 expression via a protein kinase Cdelta/p38/extracellular signal-regulated kinase pathway in phorbol myristate acetate-induced THP-1 macrophages. Clin Exp Pharmacol Physiol 38:11–18
Yu WY, Kan WJ, Yu PX et al (2012) Anti-inflammatory effect and mechanism of artemisinin and dihydroartemisinin. China J Chin Mater Med 37:2618–2621. (in Chinese)
Wu B, Hu K, Li S et al (2012) Dihydroartiminisin inhibits the growth and metastasis of epithelial ovarian cancer. Oncol Rep 27:101–108
Kitamura T, Qian B-Z, Pollard JW (2015) Immune cell promotion of metastasis. Nat Rev Immunol 15(2):73–86. doi:10.1038/nri3789
Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W et al (2014) Inactivation of the PI3K p110δ breaks regulatory T cell-mediated immune tolerance to cancer. Nature 510(7505):407–411. doi:10.1038/nature13444
Sun XZ (1991) Experimental study on the immunosuppressive effects of qinghaosu and its derivative. Chin J Mod Dev Tradit Med 11:37–38 (in Chinese)
Wang JX, Tang W, Shi LP, Wan J, Zhou R, Ni J et al (2007) Investigation of the immunosuppressive activity of artemether on T-cell activation and proliferation. Br J Pharmacol 150:652–661
Yang DM, Liew FY (1993) Effects of qinghaosu (artemisinin) and its derivatives on experimental cutaneous leishmaniasis. Parasitology 106(Pt 1):7–11
Oleinika K, Nibbs RJ, Graham GJ, Fraser AR (2012) Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin Exp Immunol 171:36–45
Langroudi L, Hassan ZM, Ebtekar M, Mahdavi M, Pakravan N, Noori S (2010) A comparison of low-dose cyclophosphamide treatment with artemisinin treatment in reducing the number of regulatory T cells in murine breast cancer model. Int Immunopharmacol 10:1055–1061
Zhang LX, Liu ZN, Ye J, Sha M, Qian H, Bu XH et al (2014) Artesunate exerts an antiimmunosuppressive effect on cervical cancer by inhibiting PGE2 production and Foxp3 expression. Cell Biol Int 38:639–646
Ramacher M, Umansky V, Efferth T (2009) Effect of artesunate on immune cells in ret-transgenic mouse melanoma model. Anti Cancer Drug 20:910–917
Mohamadabadi MA, Hassan ZM, Hosseini AZ, Gholamzad M, Noori S, Mahdavi M et al (2013) Arteether exerts antitumor activity and reduces CD4 + CD25 + FOXP3 + T-reg cells in vivo. Iran J Immunol 10:139–149
Noori S, Hassan ZM (2011) Dihydroartemisinin shift the immune response towards Th1, inhibit the tumor growth in vitro and in vivo. Cell Immunol 271:67–72
Caspi R (2008) Immunotherapy of autoimmunity and cancer: the penalty for success. Nat Rev Immunol 8(12):970–976. doi:10.1038/nri2438
Schieber M, Chandel NS (2014) ROS Function in Redox Signaling and Oxidative Stress. Curr Biol 24(10):R453–R462. doi:10.1016/j.cub.2014.03.034
Chen X, Song M, Zhang B, Zhang Y (2016) Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxidative Medicine and Cellular Longevity. doi:10.1155/2016/1580967
Vieira FGK, Di Pietro PF, Boaventura BCB, Ambrosi C, Rockenbach G et al (2011) Factors associated with oxidative stress in women with breast cancer. Nutr Hosp 26(3):528–536
Mecdad AA, Ahmed MH, ElHalwagy MEA, Afify MMM (2011) A study on oxidative stress biomarkers and immunomodulatory effects of pesticides in pesticide-sprayers. Egyptian. J Forensic Sci 1:93–98. doi:10.1016/j.ejfs.2011.04.012
Brenner DR, Scherer D, Muir K, Schildkraut J, Boffetta P et al (2014) A review of the application of inflammatory biomarkers in epidemiologic. Cancer Res. doi:10.1158/1055-9965.EPI-14-0064
Acknowledgements
The authors are indebted to the Mauritius Research Council for funding drug delivery research and to Bionexx Company (Madagascar) for funding a project related to artemisinin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors collaborate with Bionexx Company (Madagascar), a supplier of artemisinin, in developing nano-based formulations.
Rights and permissions
About this article
Cite this article
Bhaw-Luximon, A., Jhurry, D. Artemisinin and its derivatives in cancer therapy: status of progress, mechanism of action, and future perspectives. Cancer Chemother Pharmacol 79, 451–466 (2017). https://doi.org/10.1007/s00280-017-3251-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3251-7