Abstract
Purpose
Trifluridine (TFT) is an antitumor component of a novel nucleoside antitumor agent, TAS-102, which consists of TFT and tipiracil hydrochloride (thymidine phosphorylase inhibitor). Incorporation of TFT into DNA is a probable mechanism of antitumor activity and hematological toxicity. The objective of this study was to examine the TFT incorporation into tumor- and white blood cell-DNA, and to elucidate the mechanism of TFT-related effect and toxicity. TFT effect on the colony formation of mouse bone marrow cells was also investigated.
Methods
Pharmacokinetics of TFT was determined in nude mice after single oral administration of TAS-102, while the antitumor activity and body weight change were evaluated in the tumor-bearing nude mice after multiple oral administrations for 2 weeks. TFT concentrations in the blood- and tumor-DNA were determined by LC/MS/MS. The colony formation was evaluated by CFU-GM assay.
Results
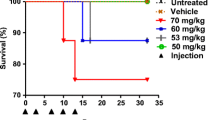
TFT systemic exposure in plasma increased dose-dependently. The tumor growth rate and body weight gain decreased dose-dependently, but TFT concentrations in the DNA of tumor tissues and white blood cells increased dose-dependently. TFT inhibited colony formation of bone marrow cells in a concentration-dependent manner.
Conclusions
A significant relationship between systemic exposure of TFT and pharmacological effects including the antitumor activity and body weight change was well explained by the TFT incorporation into DNA. TFT inhibited proliferations of mouse bone marrow cells and human colorectal carcinoma cells implanted to nude mice dose-dependently. The highest tolerable TFT exposure provides the highest antitumor activity, and the hematological toxicity may serve as a potential surrogate indicator of TAS-102 efficacy.





Similar content being viewed by others
Abbreviations
- AZT:
-
Zidovudine
- TFT:
-
Trifluridine
- C max :
-
Maximum plasma concentration
- AUC:
-
Area under plasma concentration–time curve
- AUC0–t :
-
AUC from time 0 to the time (t) of the last quantifiable concentration
- AUC0–12h :
-
AUC from time 0 to the last time point (12 h)
- TPI:
-
Tipiracil hydrochloride
- BW:
-
Body weight
- LLOQ:
-
Lower limit of quantification
- LC/MS/MS:
-
Liquid chromatography–tandem mass spectrometry
- FTY:
-
Trifluorothymine
- HPMC:
-
Hydroxypropyl methylcellulose
- PK:
-
Pharmacokinetics
- PD:
-
Pharmacodynamics
- TV:
-
Tumor volume
- RTV:
-
Relative tumor volume
- TGI:
-
Tumor growth inhibition rate
References
Basu S, Hodgson G, Katz M, Dunn AR (2002) Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 100:854–861
Dexter DL, Wolberg WH, Ansfield FJ, Helson L, Heidelberger C (1972) The clinical pharmacology of 5-trifluoromethyl-2′-deoxyuridine. Cancer Res 32:247–253
Di Maio M, Gridelli C, Gallo C, Shepherd F, Piantedosi FV, Cigolari S, Manzione L, Illiano A, Barbera S, Robbiati SF, Frontini L, Piazza E, Ianniello GP, Veltri E, Castiglione F, Rosetti F, Gebbia V, Seymour L, Chiodini P, Perrone F (2005) Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol 6:669–677
Doi T, Ohtsu A, Yoshino T, Boku N, Onozawa Y, Fukutomi A, Hironaka S, Koizumi W, Sasaki T (2012) Phase I study of TAS-102 treatment in Japanese patients with advanced solid tumours. Br J Cancer 107:429–434
Du DL, Volpe DA, Grieshaber CK, Murphy MJ Jr (1990) Effects of l-phenylalanine mustard and L-buthionine sulfoximine on murine and human hematopoietic progenitor cells in vitro. Cancer Res 50:4038–4043
Emura T, Suzuki N, Yamaguchi M, Ohshimo H, Fukushima M (2004) A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol 25:571–578
Erickson-Miller CL, May RD, Tomaszewski J, Osborn B, Murphy MJ, Page JG, Parchment RE (1997) Differential toxicity of camptothecin, topotecan and 9-aminocamptothecin to human, canine, and murine myeloid progenitors (CFU-GM) in vitro. Cancer Chemother Pharmacol 39:467–472
Fukushima M, Suzuki N, Emura T, Yano S, Kazuno H, Tada Y, Yamada Y, Asao T (2000) Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol 59:1227–1236
Hong DS, Abbruzzese JL, Bogaard K, Lassere Y, Fukushima M, Mita A, Kuwata K, Hoff PM (2006) Phase I study to determine the safety and pharmacokinetics of oral administration of TAS-102 in patients with solid tumors. Cancer 107:1383–1390
Overman MJ, Varadhachary G, Kopetz S, Thomas MB, Fukushima M, Kuwata K, Mita A, Wolff RA, Hoff PM, Xiong H, Abbruzzese JL (2008) Phase 1 study of TAS-102 administered once daily on a 5-day-per-week schedule in patients with solid tumors. Invest New Drugs 26:445–454
Parchment RE, Volpe DA, LoRusso PM, Erickson-Miller CL, Murphy MJ Jr, Grieshaber CK (1994) In vivo–in vitro correlation of myelotoxicity of 9-methoxypyrazoloacridine (NSC-366140, PD115934) to myeloid and erythroid hematopoietic progenitors from human, murine, and canine marrow. J Natl Cancer Inst 86:273–280
Rambach L, Bertaut A, Vincent J, Lorgis V, Ladoire S, Ghiringhelli F (2014) Prognostic value of chemotherapy-induced hematological toxicity in metastatic colorectal cancer patients. World J Gastroenterol 20:1565–1573
Suzuki N, Emura T, Fukushima M (2011) Mode of action of trifluorothymidine (TFT) against DNA replication and repair enzymes. Int J Oncol 39:263–270
Tanaka N, Sakamoto K, Okabe H, Fujioka A, Yamamura K, Nakagawa F, Nagase H, Yokogawa T, Oguchi K, Ishida K, Osada A, Kazuno H, Yamada Y, Matsuo K (2014) Repeated oral dosing of TAS-102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models. Oncol Rep 32:2319–2326
Temmink OH, de Bruin M, Comijn EM, Fukushima M, Peters GJ (2005) Determinants of trifluorothymidine sensitivity and metabolism in colon and lung cancer cells. Anticancer Drugs 16:285–292
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M (2007) Predictive value of chemotherapy-induced neutropenia for the efficacy of oral fluoropyrimidine S-1 in advanced gastric carcinoma. Br J Cancer 97:37–42
Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, Tsuji Y, Moriwaki T, Esaki T, Hamada C, Tanase T, Ohtsu A (2012) TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 13:993–1001
Yoshino T, Mayer R, Falcone A et al (2014) Results of a multicenter, randomized, double-blind, phase III study of TAS-102 vs placebo, with best supportive care (BSC) in patients (PTS) with metastatic colorectal cancer (MCRC) refractory to standard therapies (RECOURSE). Ann Oncol 25(suppl 2):ii114
Acknowledgments
We thank Dr. Kiyoshi Morikawa for providing the KM20C cells. All authors are employees of Taiho Pharmaceutical Co., Ltd., the company that developed TAS-102.
Conflict of interest
Authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, F., Komoto, I., Oka, H. et al. Exposure-dependent incorporation of trifluridine into DNA of tumors and white blood cells in tumor-bearing mouse. Cancer Chemother Pharmacol 76, 325–333 (2015). https://doi.org/10.1007/s00280-015-2805-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2805-9