Abstract
Purpose
Busulfan (Bu) exposure is critical for efficacy and safety. Body weight (BW), or adjusted ideal body weight (AIBW)-based dosing (WBD) algorithm, has been used in hematopoietic stem cell transplantation (HSCT). A recently completed phase 2 study revealed that 33.6 % of the subjects were under-, or over-exposed, with this WBD algorithm. This paper was to investigate Bu dosing algorithm in an attempt to improve the suboptimal Bu exposure.
Methods
Population PK modeling was conducted using the data from 207 patients. Dosing algorithm was developed based on derived covariate model of CL. Model-based simulation was conducted to assist test PK study design. A simplified CL estimation method was proposed based on the PK structure model for Bu.
Results
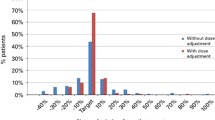
A one-compartment structure model adequately described the PK profile of Bu following an IV infusion. BSA best described the inter-individual variability of CL. The proposed dosing algorithm was dose (mg) = (31.7 × BSA − 11.6) × target AUC [µM min]/1,000. With this dosing algorithm, 14.3 % patients could be under- or over-exposed. A test PK study with reduced study duration and three PK samples can provide as nearly as good an estimate of CL compared to 12 PK samples on two different occasions.
Conclusion
The proposed dosing algorithm can significantly improve the sub-exposure of Bu. A shortened test PK study duration with reduced PK samples can provide as near as good estimate for Bu CL. A simplified CL estimation method is valid.



Similar content being viewed by others
References
Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D et al (2008) High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant 14:220–228
Andersson BS, de Lima MJ, Saliba RM, Shpall EJ, Popat U, Roy Jones R et al (2011) Pharmacokinetic dose guidance of IV busulfan with fludarabine with allogeneic stem cell transplantation improves progression free survival in patients with AML and MDS; Results of a randomized phase III study. Blood 118:892
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al (2002) Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 8:477–485
Dean RM, Pohlman B, Sweetenham JW, Sobecks RM, Kalaycio ME, Smith SD et al (2010) Superior survival after replacing oral with intravenous busulfan in autologous stem cell transplantation for non-Hodgkin lymphoma with busulfan, cyclophosphamide and etoposide. Br J Haematol 148:226–234
Lee JH, Choi SJ, Lee JH, Kim SE, Park CJ, Chi HS et al (2005) Decreased incidence of hepatic veno-occlusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan + cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol 84:321–330
Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W et al (2002) Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 8:493–500
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS et al (2000) Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 6:548–554
Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ et al (2002) Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 8:145–154
Russell JA, Kangarloo SB (2008) Therapeutic drug monitoring of busulfan in transplantation. Curr Pharm Des 14:1936–1949
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R et al (2004) Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 104:857–864
Nguyen L, Leger F, Lennon S, Puozzo C (2006) Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol 57:191–198
Russell JA, Kangarloo SB, Williamson T, Chaudhry MA, Savoie ML, Turner AR et al (2013) Establishing a target exposure for once-daily intravenous busulfan given with fludarabine and thymoglobulin before allogeneic transplantation. Biol Blood Marrow Transplant 19:1381–1386
Gibbs P, Gooley T, Corneau B, Murray G, Stewart P, Appelbaum FR et al (1999) The impact of obesity and disease on busulfan oral clearance in adults. Blood 93:4436–4440
Lill M, Costa LJ, Yeh RF, Lim S, Stuart R, Waller EK et al (2013) Pharmacokinetic-directed dose adjustment is essential for intravenous busulfan exposure optimization. Biol Blood Marrow Transplant 19(2 Supplement):S132
McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH (2014) Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clin Cancer Res 20:754–763
Acknowledgments
The authors thank Seattle Cancer Care Alliance that conducted the PK Study. The authors also thank Ms. Paula Kaptur for her assistance during the manuscript preparation.
Conflict of interest
The study was sponsored by Otsuka Pharmaceutical Development and Commercialization, Inc. All authors were Otsuka full time employers when this work was completed. X. Wang. K. Kato, Y. Wang, C. Gallo conducted the analysis, interpreted the results, and prepared the manuscript. K. Kato, E. Rock, and E. Armstrong designed and supervised the clinical study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Kato, K., Le Gallo, C. et al. Dosing algorithm revisit for busulfan following IV infusion. Cancer Chemother Pharmacol 75, 505–512 (2015). https://doi.org/10.1007/s00280-014-2660-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2660-0