Abstract
Purpose
This phase I study investigated the safety, dose-limiting toxicity, and efficacy in three cohorts all treated with the mTOR inhibitor everolimus that was delivered (1) in combination with 5-fluorouracil with leucovorin (5-FU/LV), (2) with mFOLFOX6 (5-FU/LV + oxaliplatin), and (3) with mFOLFOX6 + panitumumab in patients with refractory solid tumors.
Methods
Patients were accrued using a 3-patient cohort design consisting of two sub-trials in which the maximum tolerated combination (MTC) and dose-limiting toxicity (DLT) of everolimus and 5-FU/LV was established in Sub-trial A and of everolimus in combination with mFOLFOX6 and mFOLFOX6 plus panitumumab in Sub-trial B.
Results
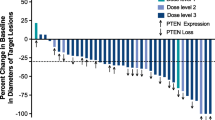
Thirty-six patients were evaluable for toxicity, 21 on Sub-trial A and 15 on Sub-trial B. In Sub-trial A, DLT was observed in 1/6 patients enrolled on dose level 1A and 2/3 patients in level 6A. In Sub-trial B, 2/3 patients experienced DLT on level 1B and subsequent patients were enrolled on level 1B-1 without DLT. Three of six patients in cohort 2B-1 experienced grade 3 mucositis, and further study of the combination of everolimus, mFOLFOX6 and panitumumab was aborted. Among the 24 patients enrolled with refractory metastatic colorectal cancer, the median time on treatment was 2.7 months with 45 % of patients remaining on treatment with stable disease for at least 3 months.
Conclusions
While a regimen of everolimus in addition to 5-FU/LV and mFOLFOX6 appears safe and tolerable, the further addition of panitumumab resulted in an unacceptable level of toxicity that cannot be recommended for further study. Further investigation is warranted to better elucidate the role which mTOR inhibitors play in patients with refractory solid tumors, with a specific focus on mCRC as a potential for the combination of this targeted and cytotoxic therapy in future studies.

Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Courtney KD, Corcoran RB, Engelman JA (2010) The PI3 K pathway as drug target in human cancer. J Clin Oncol 28:1075–1083
Houghton PJ (2010) Everolimus. Clin Cancer Res 16:1368–1372
Samuels Y, Wang Z, Bardelli A et al (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304:554
Bradshaw-Pierce EL, Pitts TM, Kulikowski G et al (2013) Utilization of quantitative in vivo pharmacology approaches to assess combination effects of everolimus and irinotecan in mouse xenograft models of colorectal cancer. PLoS ONE 8:e58089
Chu C, Noel-Hudson MS, Boige V et al (2013) Therapeutic efficiency of everolimus and lapatinib in xenograft model of human colorectal carcinoma with KRAS mutation. Fundam Clin Pharmacol 27:434–442
Altomare I, Hurwitz H (2013) Everolimus in colorectal cancer. Expert Opin Pharmacother 14:505–513
Altomare I, Bendell JC, Bullock KE et al (2011) A phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist 16:1131–1137
Ng K, Tabernero J, Hwang J et al (2013) Phase II study of everolimus in patients with metastatic colorectal adenocarcinoma previously treated with bevacizumab-, fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens. Clin Cancer Res 19:3987–3995
Wolpin BM, Ng K, Zhu AX et al (2013) Multicenter phase II study of tivozanib (AV-951) and everolimus (RAD001) for patients with refractory, metastatic colorectal cancer. Oncologist 18:377–378
Wang Q, Wei F, Li C et al (2013) Combination of mTOR and EGFR kinase inhibitors blocks mTORC1 and mTORC2 kinase activity and suppresses the progression of colorectal carcinoma. PLoS ONE 8:e73175
Wang HW, Yang SH, Huang GD et al (2014) Temsirolimus enhances the efficacy of cetuximab in colon cancer through a CIP2A-dependent mechanism. J Cancer Res Clin Oncol 140:561–571
Punt CJ, Boni J, Bruntsch U, Peters M, Thielert C (2003) Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol 14:931–937
Amado RG, Wolf M, Peeters M et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Soefje SA, Karnad A, Brenner AJ (2011) Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol 6:125–129
Grothey A, Van Cutsem E, Sobrero A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McRee, A.J., Davies, J.M., Sanoff, H.G. et al. A phase I trial of everolimus in combination with 5-FU/LV, mFOLFOX6 and mFOLFOX6 plus panitumumab in patients with refractory solid tumors. Cancer Chemother Pharmacol 74, 117–123 (2014). https://doi.org/10.1007/s00280-014-2474-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2474-0