Abstract
Purpose
Bosutinib (SKI-606), a dual Src/Abl tyrosine kinase inhibitor, is in clinical development for the treatment of patients with chronic myelogenous leukemia (CML). To support clinical development, we conducted a dose-escalation and food-effect evaluation of safety, tolerability, and pharmacokinetics (PK) of bosutinib in healthy adults.
Methods
This was a randomized, double-blind, placebo-controlled, single-ascending dose, sequential-group study of oral bosutinib. Subjects randomly received bosutinib 200, 400, 600, and 800 mg with food; 200 and 400 mg without food; or placebo. Plasma concentrations were determined by a liquid chromatography-tandem mass spectrometry assay. Non-compartmental PK analyses were performed, and power models assessed dose linearity.
Results
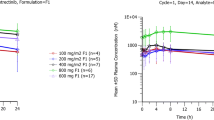
Of 55 enrolled subjects, 33 (81%) subjects had adverse events (AEs) after receiving bosutinib. Common AEs included diarrhea (39%), nausea (29%), and headache (22%). Bosutinib 200–600 mg with food was safe and well tolerated. Bosutinib exposures (C max and AUC) were linear and dose proportional from 200 to 800 mg with food. Absorption was relatively slow; median time to C max was 6 h. Apparent volume of distribution (V z/F) was 131–214 L/kg, mean apparent clearance (CL/F) was 2.25–3.81 L/h/kg, and mean terminal elimination half-life (t 1/2) was 32–39 h. Preliminary food effect assessment showed that exposure to bosutinib increased by ~2.52-fold (P = 0.002) for C max and ~2.28-fold (P = 0.002) for AUC when 200 mg bosutinib was administered with food compared with administration under fasting conditions; administration of 400 mg bosutinib with food increased AUC by ~1.5-fold (P = 0.037). Approximately 1% of administered dose was excreted in urine.
Conclusions
Bosutinib 200–600 mg with food was safe and well tolerated. Under fed conditions, bosutinib exposures were linear and dose proportional, and C max increased by ~1.5-fold. The t 1/2 supported a once-daily dosing regimen.


Similar content being viewed by others
References
Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13:513–609
Avizienyte E, Frame MC (2005) Src and FAK signaling controls adhesions fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Bio 17:542–547
Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, Gallick GE (2002) Src activation regulates anoikis in human colon tumor cell lines. Oncogene 21:7797–7807
Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602:114–130
Fizazi K (2007) The roles of Src in prostate cancer. Ann Oncol 18:1765–1773
Homsi J, Cubitt C, Daud A (2007) The Src signaling pathway: a potential target in melanoma and other malignancies. Expert Opin Ther Targets 11:91–100
Pendergast AM (2002) The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res 85:51–100
Srinivasan D, Plattner R (2006) Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res 66:5648–5655
Hantschel O, Superti-Furga G (2004) Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol 5:33–34
Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH (2003) SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res 63:375–381
Puttini M, Coluccia AM, Boschelli F, Cleris L, Marchesi E, Donella-Deana A, Ahmed S, Redaelli S, Piazza R, Magistroni V, Andreoni F, Scapozza L, Formelli F, Gambacorti-Passerini C (2006) In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl + neoplastic cells. Cancer Res 66:11314–11322
Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, Jove R (2008) SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther 7:1185–1194
Campone M, Bondarenko I, Brincat S, Epstein RJ, Munster PN, Dubois E, Martin EC, Turnbull K, Zacharchuk C (2007) Preliminary results of a phase 2 study of bosutinib (SKI-606), a dual Src/Abl kinase inhibitor, in patients with advanced breast cancer. Breast Cancer Res Treat 106: abstract 6062
Gambacorti-Passerini C, Pogliani EM, Baccarani M, Martinelli G, Kantarjian HM, Chandy M, Khoury HJ, Kim D, Brummendorf TH, Arkin S, Cortes J (2008) Bosutinib (SKI-606) demonstrates clinical activity and is well tolerated in patients with AP and BP CML and Ph + ALL. Blood 112: abstract 1101
Cortes J, Kantarjian H, Brümmendorf T, Khoury HJ, Kim D, Turkina A, Volkert A, Wang J, Arkin S, Gambacorti-Passerini C (2010) Safety and efficacy of bosutinib (SKI-606) in patients (pts) with chronic phase (CP) chronic myelogenous leukemia (CML) following resistance or intolerance to imatinib (IM). J Clin Oncol 28(Suppl 15): abstract 6502
Evans WE, Schentag JJ, Jusko WJ (1992) Principles of therapeutic drug monitoring, 3rd edn. Applied Therapeutics, Inc., Vancouver, WA
Gough K, Hutchison M, Keene ON, Byrom B, Ellis S, Lacey LF, McKellar J (1995) Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/pharmacokinetics UK joint working party. Drug Inf J 29:1039–1048
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (2000) Guidance for industry. Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. August 2000. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246 pdf 2000 [cited 2011 May 11]; Available from: URL: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070246.pdf
Abbas RA, Hug BA, Leister C, Burns J, Sonnichsen D (2011) Effect of ketoconazole on the pharmacokinetics of oral bosutinib in healthy subjects. J Clin Pharmacol. doi:10.1177/0091270010387427
Acknowledgments
This study was sponsored by Wyeth Research, which was acquired by Pfizer in October 2009. We thank all the subjects who participated in this study, as well as the study staff of the Kendle Phase 1 Unit, Utrecht, The Netherlands. Editorial/medical writing support was provided by Joseph Ramcharan, PhD, at On Assignment Clinical Research, and Kimberly Brooks, PhD, at MedErgy, and was funded by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbas, R., Hug, B.A., Leister, C. et al. A phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjects. Cancer Chemother Pharmacol 69, 221–227 (2012). https://doi.org/10.1007/s00280-011-1688-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1688-7