Abstract
Objectives
To explore the outcomes of combined transarterial chemoembolization (TACE) with sorafenib in hepatocellular carcinoma (HCC) patients with portal vein tumour thrombus (PVTT) and to establish a prognostic prediction nomogram to differentiate target patients and stratify risk.
Materials and Methods
This multicentre, retrospective study consisted of 185 consecutive treatment-naïve patients with HCC and PVTT treated with TACE plus sorafenib from three institutions between January 1st, 2012 and December 31st, 2017. The primary outcome measurement of the study was overall survival (OS). The type of PVTT was classified by the Liver Cancer Study Group of Japan. The prognostic nomogram was established based on the predictors and was performed with interval validation.
Results
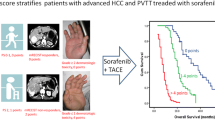
The median OS of the Vp1-3 and Vp4 groups was 12.4 months (11.7–18.9) and 8.5 months (7.6–11.2) (P = 0.00098), respectively, and there was a significant difference in the median OS between the Vp1-2 and Vp3 subgroups (16.4 months (12.2–27.9) vs. 10.9 months (8.4–18.1), P = 0.041). The multivariate Cox regression analysis suggested that tumour size, albumin-bilirubin grade, and PVTT type were independent prognostic factors. The C-index value of the nomogram based on these predictors in the entire cohort was 0.731 (0.628–0.833).
Conclusions
After the combined therapy of TACE and sorafenib, advanced HCC patients with segmental or subsegmental PVTT showed better survival than those with main PVTT. The nomogram can be applied to identify advanced HCC patients with PVTT who may benefit most from the combination treatment and be helpful for making decision in clinical practice.



Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- BCLC:
-
Barcelona clinic liver cancer
- TACE:
-
Transarterial chemoembolization
- cTACE:
-
Conventional transarterial chemoembolization
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PVTT:
-
Portal vein tumour thrombus
- IRBs:
-
Institutional review boards
- EASL:
-
European association for the study of the liver
- AASLD:
-
American association for the study of liver diseases
- CT:
-
Computed tomography
- CECT:
-
Contrast-enhanced computed tomography
- ECOG:
-
Eastern cooperative oncology group
- VEGF:
-
Vascular endothelial growth factor
- PDGFR:
-
Platelet-derived growth factor receptor
- LCSGJ:
-
Liver cancer study group of Japan
- MRI:
-
Magnetic resonance imaging
- ALBI:
-
Albumin-bilirubin
- CTP:
-
Child-turcotte-pugh
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- TTP:
-
Time to progression
- TTR:
-
Tumour response rate
- CTCAE:
-
Common terminology criteria of adverse events
- C-index:
-
Concordance index
- mRECIST:
-
Modified response evaluation criteria in solid tumors
- AEs:
-
Adverse events
- CI:
-
Confidence interval
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233(3):379–84. https://doi.org/10.1097/00000658-200103000-00012.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–55. https://doi.org/10.1016/S0140-6736(11)61347-0.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–62. https://doi.org/10.1056/NEJMra1713263.
Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–53. https://doi.org/10.1053/j.gastro.2015.12.041.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. https://doi.org/10.1056/NEJMoa0708857.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. https://doi.org/10.1016/S1470-2045(08)70285-7.
Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: beyond the known frontiers. World J Gastroenterol. 2019;25(31):4360–82. https://doi.org/10.3748/wjg.v25.i31.4360.
Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903. https://doi.org/10.1016/j.jhep.2019.01.013.
Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Konigsberg R, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263(2):590–9. https://doi.org/10.1148/radiol.12111550.
Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18(2):413–20. https://doi.org/10.1245/s10434-010-1321-8.
Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. https://doi.org/10.1186/1471-230X-13-60.
Dufour JF, Hoppe H, Heim MH, Helbling B, Maurhofer O, Szucs-Farkas Z, et al. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist. 2010;15(11):1198–204. https://doi.org/10.1634/theoncologist.2010-0180.
Kudo M. Proposal of primary endpoints for tace combination trials with systemic therapy: lessons learned from 5 negative trials and the positive TACTICS trial. Liver Cancer. 2018;7(3):225–34. https://doi.org/10.1159/000492535.
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–9. https://doi.org/10.1080/02841850801958890.
Abou-Alfa GK. TACE and sorafenib: a good marriage? J Clin Oncol. 2011;29(30):3949–52. https://doi.org/10.1200/JCO.2011.37.9651.
Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117–27. https://doi.org/10.1016/j.ejca.2011.05.007.
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29(30):3960–7. https://doi.org/10.1200/JCO.2011.37.1021.
Zhao Y, Wang WJ, Guan S, Li HL, Xu RC, Wu JB, et al. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013;24(7):1786–92. https://doi.org/10.1093/annonc/mdt072.
Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269(2):603–11. https://doi.org/10.1148/radiol.13130150.
Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2019. https://doi.org/10.1136/gutjnl-2019-318934.
Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib–a retrospective controlled study. Radiology. 2014;272(1):284–93. https://doi.org/10.1148/radiol.14131946.
Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–91. https://doi.org/10.1016/j.jhep.2018.11.029.
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50. https://doi.org/10.1002/hep.29913.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018; 69(1):182–236. doi:10.1016/j.jhep.2018.03.019.
Ikai I, Arii S, Okazaki M, Okita K, Omata M, Kojiro M, et al. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res. 2007;37(9):676–91. https://doi.org/10.1111/j.1872-034X.2007.00119.x.
Lee IC, Hung YW, Liu CA, Lee RC, Su CW, Huo TI, et al. A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma. Liver Int. 2019;39(9):1704–12. https://doi.org/10.1111/liv.14194.
Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4(9):721–30. https://doi.org/10.1016/S2468-1253(19)30178-5.
Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–66. https://doi.org/10.1111/liv.12818.
Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–8. https://doi.org/10.1016/j.jhep.2016.01.012.
Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–75. https://doi.org/10.1016/S2468-1253(17)30156-5.
Friemel J, Rechsteiner M, Frick L, Bohm F, Struckmann K, Egger M, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21(8):1951–61. https://doi.org/10.1158/1078-0432.CCR-14-0122.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–38. https://doi.org/10.1055/s-2007-1007122.
Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103(9):1027–36. https://doi.org/10.1161/CIRCRESAHA.108.181115.
Tekkesin N, Taga Y, Sav A, Almaata I, Ibrisim D. Induction of HGF and VEGF in hepatic regeneration after hepatotoxin-induced cirrhosis in mice. Hepatogastroenterology. 2011;58(107–108):971–9.
Han G, Berhane S, Toyoda H, Bettinger D, Elshaarawy O, Chan AWH, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response-based approach. Hepatology. 2019. https://doi.org/10.1002/hep.31022.
Zhong BY, Ni CF, Ji JS, Yin GW, Chen L, Zhu HD, et al. Nomogram and artificial neural network for prognostic performance on the albumin-bilirubin grade for hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol: JVIR. 2019;30(3):330–8. https://doi.org/10.1016/j.jvir.2018.08.026.
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–8. https://doi.org/10.1200/JCO.2014.57.9151.
Acknowledgements
None
Funding
This study was supported by the National Natural Science Foundation of China (81901847) (81771945) (81971713), the Jiangsu Medical Innovation Team (CXTDB2017006), the Natural Science Foundation of Jiangsu Province (BK20190177), the Natural Science Foundation of Zhejiang Province (LZ18H180001), the Suzhou Science and Technology Youth Plan (KJXW2018003) and “Six One Projects” for High-level Health Personnel in Jiangsu Province (LGY2018077). Funding source had no involvement in the financial support for the conduct of the research and preparation of the article.
Author information
Authors and Affiliations
Contributions
All authors contributed to review and critical revision of the manuscript and approved the final version of the manuscript. CFN, ZPY, LZ, JHS, ZHH, and BYZ contributed to the study concept and design, LZ, ZHH, BYZ, PH, SZ, MJY, GHZ, WSW, ZL, and XLZ contributed to acquisition of data, LZ, ZHH, and BYZ contributed to analysis and interpretation of data, LZ, ZHH, and BYZ contributed to statistical analysis, LZ, JHS, ZHH, and BYZ contributed to drafting of the manuscript. The corresponding author had full access to all of the data and took full responsibility for the veracity of the data and the statistical analyses.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
The requirement to obtain informed consent was waived due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Sun, JH., Hou, ZH. et al. Prognosis Nomogram for Hepatocellular Carcinoma Patients with Portal Vein Invasion Undergoing Transarterial Chemoembolization Plus Sorafenib Treatment: A Retrospective Multicentre Study. Cardiovasc Intervent Radiol 44, 63–72 (2021). https://doi.org/10.1007/s00270-020-02579-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02579-2