Abstract
In recent guidelines of international societies, the most frequent indication for treatment after chronic type B aortic dissection (cTBAD) is aneurysmal dilatation. Endovascular repair is recommended in patients with moderate to high surgical risk or with contraindications to open repair. During the last decade, many advances have been made in the field of endovascular techniques and devices. The aim of this article is to address the current status of endoluminal techniques for the management of cTBAD including standard thoracic endovascular repair, new devices, fenestrated and branched abdominal aortic devices and false lumen occlusion techniques.
Similar content being viewed by others
Introduction
Guidelines on the treatment of aortic dissection have traditionally supported that uncomplicated type B aortic dissection (TBAD) is treated by best medical treatment (BMT) [1, 2]. However, about 25 to 50% of patients who survive the acute phase will require open repair or thoracic endovascular aortic repair (TEVAR) during the chronic phase [3, 4].
The INSTEAD XL study showed improved survival and delayed disease progression of survivors of TBAD who underwent TEVAR in addition to BMT during the subacute phase (14–90 days) [5]. A recent systematic review [6] highlighted that secondary interventions after BMT ranged between 9.0% and 40.6% in patients with TBAD. The lack of follow-up data for conservatively treated patients, presence of heterogeneity in patients and absence of consensus reporting standards for TEVAR are obstructing the interpretation of outcomes [7].
No randomized controlled trial exists comparing open surgical repair (OSR) and TEVAR for cTBAD treatment. In a systematic review by Kamman et al. [6], mortality of TEVAR for cTBAD was favorable compared to OSR. Another recent study demonstrated that TEVAR for cTBAD even in complicated cases was safe and effective. While aortic remodeling was favorable proximal to the coeliac artery after TEVAR, the low rate of distal false lumen thrombosis warranted further imaging surveillance [8].
TEVAR for aortic dissection started 20 years ago [9] and is still developing with novel techniques and devices. The aim of this article is to address the current status of endoluminal techniques for the management of cTBAD.
Risk Factors for Late Aortic Events in Patients with Uncomplicated TBAD
TEVAR is well accepted for patients with acute and chronic TBAD. Real world data attempt to fill the gap of potential risk factors associated with worse outcomes and may identify patients who benefit from TEVAR [4, 6].
Capoccia et al. [10] suggested that rapid expansion (> 1 cm/year), critical diameter (> 5.5 cm) and refractory pain may be indications for TEVAR in cTBAD. Kaji et al. [11] categorized predictors of adverse aortic events in patients with uncomplicated TBAD into clinical (age < 60 years of age; white race; heart rate > 60/min; Marfan syndrome), laboratory (FDP level > 20 μg/ml; peak CRP level > 9.6 mg/dL) and imaging findings [aortic diameter > 40 mm, patent or partially thrombosed FL; one entry tear; intimal tear at inner curvature; elliptic configuration of FL; large entry tear (> 10 mm)]. Recently, Matsushita et al. [12] identified predictors for late aortic events in cTBAD: initial aortic diameter > 40 mm, FL diameter larger than TL diameter, ulcer-like projection and age > 70 years.
Indications for Endovascular Intervention in cTBAD
In recent guidelines [1, 2, 13,14,15], the most frequent indication for treatment after cTBAD is the aneurysmal dilatation (Table 1). TEVAR is recommended in patients with moderate to high surgical risk or with contraindications to open repair.
TEVAR requires appropriate anatomy, including
-
Adequate access compatible with the required introduction systems
-
Aortic inner diameter of the un-dissected Aorta in the proximal landing zone in the range of 16–42 mm
-
≥ 20 mm of landing zone length proximal to the primary entry tear
Landing the proximal end of the device in dissected tissue increases the risk of new septal tears, rupture and retrograde dissection. Cautious should be given in severe aortic angulation > 60°, or if there is significant calcium or thrombus, additional neck length may be required.
Current Status of Endovascular Techniques for the Management of cTBAD
Since 1999, when Nienaber et al. [9] published their experience on nonsurgical reconstruction of thoracic aortic dissection by TEVAR, many devices and techniques have been developed. The fundamental concept of TEVAR in cTBAD is to cover the proximal entry tear, to redirect flow to the TL, and to achieve thrombosis and regression of the FL. Longer aortic coverage to the celiac artery to cover distal tears increases the clinical success rate but also the risk of spinal cord ischemia [16].
Oversizing in the Proximal Landing Zone
Recommendations on the degree of oversizing differ from manufacturer to manufacturer, ranging between 4 and 32% [17]. While an increased risk of retrograde type A dissection (rTAAD) has been reported in cases of oversizing > 10%, its occurrence remains rare (1.6%). As proximal landing is in a healthy undissected aortic segment, it is our practice to apply standard oversizing of 10–20%. Another risk factor regarding the incidence of rTAAD after TEVAR is the landing zone; rTAAD is 2.7% in zone 2, 1.0% in zone 3 and 1% in zone 4 [17].
-
A.
Standard TEVAR
Various stent grafts have been used in the last decade for the treatment of cTBAD (Table 2).
Valiant Navion™ (Medtronic Ave, Inc, Santa Rosa, Calif) is a lower-profile evolution of the company’s Valiant™ thoracic stent graft. The Valiant system has been assessed in the Virtue Registry [18] which was a prospective, non-randomized, multicenter European Clinical Registry. The principle clinical findings suggested that TEVAR was able to provide good protection from aortic-related death in the midterm, but with a high rate of aortic reintervention [18].
RELAY Pro (Bolton Medical, Sunrise Florida, USA) has lower profile and improved pushability and visibility compared to the previous Relay Plus stent graft system, which was assessed in RESTORE and RESTORE II studies [19, 20], showing safety and effectiveness in patients with types A or B acute or chronic aortic dissections in terms of survival and low morbidity.
The Zenith TX2 Dissection Endovascular Graft (Cook Medical, Bjaeverskov, Denmark) with Pro-Form (Fig. 1) is a one-piece tubular endovascular graft that for acute and chronic aortic dissections. The Zenith Dissection Endovascular Stent is an uncovered large diameter self-expanding stent and may be used as a distal extension in order to expand the TL [21].
The GORE® (W.L. Gore & Associates, Flagstaff, AZ, USA) conformable TAG® Thoracic Endoprosthesis was assessed in a multicenter clinical trial of TEVAR in the descending thoracic aorta [22]. This study confirmed treatment advantages for TEVAR when compared with literature-based results of open repair in terms of survival [22]. Recently, the GORE TAG Conformable Thoracic Stent Graft with active control system has been approved; the active control system allows bending the proximal part of the stent graft during deployment in order to minimize the ``bird peak'' phenomenon.
-
B.
Extending the landing zone proximal to LSA
-
1.
Custom-made devices
There is rarely sufficient seal zone distally to the left subclavian artery (LSA) in cTBAD, which frequently requires LSA-coverage and LSA-debranching. A proximal landing zone length of 20 mm is desired, although shorter landing zones are tolerable in Ishimaru zone 2 may as long as it is non-dissected. Cervical debranching or a chimney graft for the left common carotid artery (LCCA) can further extend the landing zone to the level of the innominate artery (IA). However, it is not recommended to land in the ascending aorta due to the risk of rTAAD.
The Valiant Mona LSA Thoracic Stent Graft System (Medtronic, Santa Rosa, Calif) consists of a main stent graft (MSG) and a branch stent graft (BSG) designed to maintain LSA patency when implanted in zone 2 of the aortic arch [24]. However, there are insufficient data for the safety and efficacy of this device in patients with TBAD as it has been assessed in aneurysms [23].
The W. L. Gore Thoracic Branch Endoprosthesis (W.L. Gore & Associates, Flagstaff, AZ, USA) is a single-branch device designed for either zone 0 or zone 2 deployment. There are also insufficient data for the safety and efficacy of this device in patients with TBAD, although it has been assessed in aneurysms [24].
The Cook Zenith (Cook Medical, Bjaeverskov, Denmark) fenestrated arch graft is a custom-made device (CMD) and may contain up to one scallop and one fenestration for perfusion of aortic arch vessels landing in zones 0–2 (Fig. 2). The delivery system is precurved and uses diameter reducing ties and a spiralizing wire on the central cannula to ensure rotational control as well as a preloaded catheter for fenestrations to allow a through-and-through wire from left brachial access to safely align the fenestration to the target vessel.
The Relay stent graft from Bolton (Bolton Medical, Sunrise Florida, USA) can be used as a CMD with a proximal scallop in order to remain flow to aortic branches when deployed in zone 1 or 2 [25].
Similarly, scalloped or fenestrated physician-modified endovascular grafts (PMEGs) for zone 2 TEVAR may be used even in patients with cTBAD. Trubert et al. [26] showed that scalloped or single-fenestrated PMEGs for the LSA appear to be durable and safe in the midterm. Combined with low periprocedural morbidity and mortality, these results suggest that this approach can be considered as an off-label alternative to extend proximal seal to zone 2 for TEVAR [26].
The best-known devices for branched TEVAR such as the Cook Zenith inner-branched arch endograft and the Terumo Aortic Relay double-branch endoprosthesis are neither approved for commercial use. However, the Endospan Nexus aortic archstent graft has gained CE mark recently [27], which is an ePTFE off-the-shelf system for endovascular treatment of pathologies extending or involving the aortic arch. Not being commercially available does not necessarily mean that these grafts are not recommended. Recent expert consensus papers of the ESVES and EACTS [28] have recommended their use in high-risk patients with aortic arch pathology. Recommendation 30: endovascular aortic arch repair in zone 0 should be considered in patients unfit for open surgery and with a suitable anatomy. Class IIA, Level B. However, we generally avoid using branched arch endografts in native ascending aorta in TBAD as long as other options exist. Patients with previous dissections appear at higher risk of retrograde TAAD compared to patients with atherosclerotic aneurysm.
-
2.
Chimney TEVAR
Chimney technique has mainly been used in urgent cases, when CMDs were not available or surgical revascularization was not amendable in patients with aortic aneurysms. However, evidence from Bosier et al. [29] and Mangialardi et al. [30] reported that Chimney TEVAR techniques can be also used with good outcomes in patients with TBAD.
Whether aortic arch vessels in endovascular technique are managed by debranching, chimney technique or branched/fenestrated endografts depend widely on availability of devices, technique and experience. Patient factors and anatomical considerations come into play, so that the authors’ recommendation for dedicated fenestrated/branched endografts as a first choice remains a personal choice.
-
C.
Management of the distal zone of TBAD
Besides coverage of the proximal entry tear, TEVAR may seal further distal entries along its stent graft length reducing false lumen perfusion. Further distal endovascular repair using endovascular techniques is required in patients, who do not have a sealing option in the false lumen of the descending thoracic aorta due to diameter or in patients, who develop aneurysms of the abdominal aorta that exceed the recommended treatment threshold. False lumen thrombosis depends on the length of coverage that may be extended to the celiac artery and the origin of segmental arteries arising from the false lumen [31, 32].
Another important issue regarding the management of distal zone of TBAD is the stent graft-induced new entry (SINE) which is defined as a “new tear caused by the stent graft itself, excluding those created by natural disease progression or any iatrogenic injury from endovascular manipulation [33]. SINE has been increasingly being observed after TEVAR with incidence reaching up to 25%, especially for TBAD (Fig. 3). Distal SINE could develop into a patent false lumen with subsequent aneurysmal expansion and possible rupture. The most important risk factor for distal SINE appears to be excessive oversizing of the distal stent graft relative to the smaller true lumen that may result up to > 60% in comparison with the distal true lumen.
-
1.
Fenestrated/Branched Endovascular Aortic Aneurysm Repair
Fenestrated and branched endovascular aortic repair (F/B-EVAR) may be required in patients, who do not have a sealing option in the false lumen of the descending thoracic aorta due to diameter or in patients, who develop aneurysms of the abdominal aorta that exceed the recommended treatment threshold.
Kitagawa et al. [34] showed that F/B-EVAR is a feasible option for patients with cTBAD in order to treat false lumen back flow and abdominal aortic dilatation. Recently, Oikonomou et al. [35] reported the midterm outcomes of patients treated with F/B-TEVAR for postdissection TAAA. They showed that this approach is feasible and associated with low peri-operative mortality and peri-operative morbidity. Recently, our group reported excellent technical success rate of F/BEVAR for the treatment of postdissection aneurysm and favorable 1-year outcomes in terms of mean aneurysm diameters decrease and high false lumen thrombosis rate (92%) [36] (Fig. 4).
-
2.
Provisional Extension To Induce Complete Attachment Technique (PETTICOAT)
The PETTICOAT technique consists of TEVAR with proximal tear coverage combined with a distal bare metal stent in order to reinforce the TL without covering side branches [37]. This technique was described in acute TBAD in order to achieve favorable remodeling during follow-up [38, 39]. Recently, Kazimierczak et al. [40] used this technique in a limited series of patients with cTBAD reporting favorable results when PETTICOAT was combined with covered stents in the iliac arteries. However, the remodeling capacity of a chronic dissection is limited and additional uncovered stents crossing vital reno-visceral side branches may complicate treatment with F/B-EVAR.
-
3.
Streamliner Multilayer Flow Modulator (SMFM)
A less widespread device is the Streamliner Multilayer Flow Modulator (SMFM: Cardiatis, Isnes, Belgium), which is a self-expandable braided stent interconnected in layers permitting a porosity of ~ 65%. This technology is supposed to promote thrombus formation in the aneurysm sac while maintaining the blood perfusion into the involved branches [41, 42]. A recent global registry highlighted that the SMFM may be an option for management of aortic dissection [42]. Other studies suggest that the proposed treatment mechanism of SMFM may not be effective in aneurysmal disease [41].
-
D.
False lumen occlusion techniques
Complete false lumen thrombosis is only achieved in almost half of the patients after standard TEVAR by covering the proximal part of the dissected aorta [38]. Studies have suggested that thrombosis of the false lumen may be an independent predictor of no further growth [43], while the false lumen patency may be an independent factor of poor survival in cTBAD [44]. Flow to and pressurization of the FL are thought to contribute to further aneurysmal dilatation and rupture [45].
A variation in solid and liquid endovascular materials has been used to embolize the false lumen with varying success since Loubert et al. first published their report in 2003 [46, 47]. Techniques using more dedicated materials manufactured as custom-made devices (CMD) for false lumen occlusion are the candy-plug technique and the Knickerbocker technique [48, 49].
-
1.
Candy plug technique
Since we [48] described the candy-plug technique, in 2013 several designs of candy-plug have been used as CMD from Cook (Cook Medical, Bjaeverskov, Denmark). For candy-plug placement, the FL should preferably be catheterized at the level of iliac arteries and over an extra stiff Lunderquist wire the candy-plug is placed into the false lumen with the same distal level as the true lumen stent graft proximal to the CA. This technique occludes the FL proximal to the renovisceral segment to preserve flow to reno-visceral arteries while thoracic stent graft is placed into the true lumen to the level of the celiac artery (Fig. 5). In 2017, Rohlffs et al. [50] showed a high technical success rate of 100% and aortic remodeling of 70% in chronic aortic dissection. Recently, the early outcomes of second generation candy-plug (CP II) (Cook Medical, Bjaeverskov, Denmark) (Fig. 6) have been presented, showing that this device reduces the number of procedural steps (self-closing fabric channel that obviates the need for separate occlusion of its center) and offers good seal, with low morbidity (only 2 patients with minor complications out of 14) and mortality (7%) and a high rate of aortic remodeling (88%) [51]. An important issue is the selection of correct size device. For that purpose, the operator measures the largest diameter of the FL 1 cm above the celiac trunk on the preoperative computed tomography scan; the oversize of the CP II diameter should be 10% to 30%. The CP II should be positioned always with distal alignment to the true lumen stentgraft. Our group has now used > 50 candy-plugs and continued to see promising results (Fig. 7).
-
2.
The Knickerbocker technique
This technique does not require access of the false lumen. The basis of the technique is to dilate a large diameter stent graft in the middle part of stent graft covered area at the distal descending aorta. A compliant balloon forcefully dilates a short bulgeous segment of the double tapered stent graft, causing the rupture of the dissection membrane and extension of the stent graft into the false lumen. The early outcomes of these techniques have been encouraging [49] (Fig. 8).
-
3.
Coils, plugs, onyx or glue
A systematic review of the literature [47] highlighted that embolization of false lumen even with the combination of coils, plugs, onyx and glue promotes good outcomes in terms of remodeling. Recently, Pellenc et al. [52] demonstrated that embolization of the FL of chronic aortic dissections is technically feasible with a low morbidity rate. The FL thrombosis was observed in the majority of case and promoted favorable thoracic aortic remodeling.
Discussion
Before the endovascular era, E. Stanley Crawford commented on aortic dissection that “No patient should be considered cured of the disease” [53]. Since then, development of devices and techniques has allowed treatment of cTBAD with good early- and long-term outcomes. Thus, TEVAR has been an effective treatment strategy in TBAD [54], showing good remodeling of the aorta which has been described as the expansion of true lumen and thrombosis/regression of false lumen induced by successful entry closure with TEVAR. Recently, Watanabe et al. [32] suggested that aortic remodeling after TEVAR is a significant prognostic factor for better long-term results for TBAD. In particular, the interventions to the distal part of the dissection and/or the embolization of FL have led to favorable outcomes with the reduction in aneurysm diameters and the successful false lumen thrombosis [47].
Patent false lumen and aortic diameter themselves have been associated with aortic enlargement [55], while anatomic complexities such as acute aortic curvature and covered side branches were associated with endoleaks [56]. Recently, Sharafuddin et al. [57] introduced a new false lumen-based classification schema for endoleaks occurring after endovascular therapy of type B aortic dissection that may be used in the near future in order to better describe aortic remodeling during follow-up period.
An important issue of TEVAR remains the incidence of stroke. LSA coverage has been identified as a risk factor for stroke. Systematic review has reported an overall stroke rate of 7.4% for TEVAR following LSA coverage versus 4.0% in TEVAR performed distal to the LSA with zone 3 or 4 deployment (p < 0.0001) [58]. In a very recent meta-analysis, it was demonstrated that revascularization of the LSA is associated with decreased risks of cerebrovascular accident, spinal cord ischemia and left upper limb ischemia after TEVAR [59]. However, the rate of local complications after LSA revascularization may be significant leading to higher re-intervention rate and morbidity [60].
Another potential cause of stroke in TEVAR procedures is air embolism which is a potentially underappreciated problem of aortic endografting, especially in the proximal segments of the aorta. The additional use of carbon dioxide should be considered as a standard flush technique for aortic stent grafts, especially in those implanted in proximal aortic segments, to reduce the risk of air embolism and stroke [61, 62].
In a recent systematic review of the literature, it was shown that spinal cord ischemia risk remains low in patients treated with endovascular approach for TBAD, particularly in centers with ≥ 40 caseload [63]. There is a thin balance between benefit and harm; thus, more extensive stent graft coverage appears to improve thoracic aortic remodeling after TEVAR; however, the clinician should balance the benefit of extensive stent graft coverage and its related risk of spinal cord ischemia [64].
In a review of the literature, Canaud et al. [65] suggested that whereas distal SINE is relatively frequent, if it does occur, the complication can be generally treated with additional TEVAR with a good outcome, while the main determinant of SINE seems to be excessive distal oversizing. A recent meta-analysis on distal SINE by D’cruz et al. [66] demonstrated that chronic TBAD and an excessive distal oversizing ratio are both positively and independently associated with the incidence of dSINE tears in TBAD. Lortz et al. [67] highlighted that the use of tapered stent grafts might be beneficial for patients with high expected distal oversize, while other physicians suggest a distal to proximal endograft implantation sequence.
Conclusion
During the last decade, many endovascular devices and techniques have been developed in order to treat patients with cTBAD. Complexity and variation in disease as well as the difference in endovascular techniques make it difficult to draw valid conclusions about the place of TEVAR and its preferred technique. The use of reporting standards and randomized controlled trials are warranted to better understand the role of endovascular techniques in cTBAD.
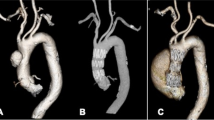
A Preoperative computed tomography angiography of a chronic type B dissection case; B intra-operative angiography of a Zenith stent graft with a fenestration (blue arrow) for the left subclavian artery and a scallop for the left common carotid artery. C Postoperative computed tomography angiography showing the false lumen thrombosis and the presence of candy-plug (red arrow)
Stent graft-induced new entry (SINE) is defined as a “new tear caused by the stent graft itself, excluding those created by natural disease progression or any iatrogenic injury from endovascular manipulation,” and has been increasingly being observed after thoracic endovascular aortic repair especially in type B aortic dissection (blue arrow)
Fenestrated endovascular aneurysm repair (F-EVAR) in a chronic type B aortic dissection; A Intra-operatively, the device has been orientated according to the circle signs from CT fusion system that shows the target vessels (CT: coeliac trunk, SMA: superior mesenteric artery; RRA: right renal artery; LRA: left renal artery). B The postoperative computed tomography angiography of this patient treated with thoracic stent graft, carotid subclavian bypass (arrow) for the proximal part and F-EVAR for the distal part of the disease
A Computed tomography angiography from endovascular repair with a thoracic stent graft in the true lumen and a candy-plug device in the false lumen (blue arrows); B axial view of distal part of the endograft and the thrombosed false lumen; C: sagittal view of the candy-plug device and the thrombosed false lumen
A Preoperative computed tomography angiography (CTA) of a patient with chronic type B aortic dissection; B postoperative CTA showing the endovascular treatment with a thoracic stent graft in the true lumen, a vascular plug for the occlusion of the left subclavian artery (blue arrow) and a candy-Plug II (red arrow) in the false lumen
Postoperative computed tomography angiography of a patient treated with the Knickerbocker technique after frozen elephant trunk repair. The short bulgeous segment of the stent graft is shown with the blue arrows, having caused the rupture of the dissection membrane and extension of the stent graft into the false lumen
Change history
04 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00270-021-02949-4
References
Riambau V, Böckler D, Brunkwall J, et al. Editor's choice-management of descending thoracic aorta diseases: clinical practice guidelines of the European society for vascular surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:4–52.
Hiratzka LF, Bakris GL, Beckman JA, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:266–369.
Hughes GC. Management of acute type B aortic dissection. ADSORB Trial J Thorac Cardiovasc Surg. 2015;149:S158–S162162.
Luebke T, Brunkwall J. Type B aortic dissection: a review of prognostic factors and meta-analysis of treatment options. Aorta. 2014;2:265–78.
Nienaber CA, Kische S, Rousseau H, et al. INSTEAD-XL trial. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407–16. https://doi.org/10.1161/CIRCINTERVENTIONS.113.000463.
Kamman AV, de Beaufort HW, van Bogerijen GH, et al. Contemporary management strategies for chronic type B aortic dissections: a systematic review. PLoS ONE. 2016;11(5):e0154930. https://doi.org/10.1371/journal.pone.0154930.
Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;42:632–47. https://doi.org/10.1016/j.ejvs.2011.08.009.
Chou HW, Chan CY, Chang CH, et al. Comparisons of aortic remodelling and outcomes after endovascular repair of acute and chronic complicated Type B aortic dissections. Interact Cardiovasc Thorac Surg. 2018;27:733–41. https://doi.org/10.1093/icvts/ivy167.
Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. New Engl J Med. 1999;340:1539–45. https://doi.org/10.1056/NEJM199905203402003.
Capoccia L, Riambau V. Current evidence for thoracic aorta type B dissection management. Vascular. 2014;22:439–47. https://doi.org/10.1177/1708538113504400.
Kaji S. Update on the therapeutic strategy of type B aortic dissection. J Atheroscler Thromb. 2018;25:203–12. https://doi.org/10.5551/jat.RV17017.
Matsushita A, Tabata M, Mihara W, et al. Risk score system for late aortic events in patients with uncomplicated type B aortic dissection. J Thorac Cardiovasc Surg. 2019;S0022–5223(19):31276.
Erbel R, Aboyans V, Boileau C, et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. the task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–926.
JCS Joint Working Group. Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011): digest version. Circ J. 2013;77:789–828 .
Appoo JJ, Bozinovski J, Chu MW, et al. Canadian Cardiovascular Society/Canadian Society of Cardiac Surgeons/Canadian Society for Vascular Surgery Joint Position Statement on Open and Endovascular Surgery for ThoracicAortic Disease. Can J Cardiol. 2016;32:703–13. https://doi.org/10.1016/j.cjca.2015.12.037.
Wang GJ, Cambria RP, Lombardi JV, et al. Thirty-day outcomes from the Society for Vascular Surgery Vascular Quality Initiative thoracic endovascular aortic repair for type B dissection project. J Vasc Surg. 2019;69(3):680–91. https://doi.org/10.1016/j.jvs.2018.06.203.
Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg. 2014;260:389–95. https://doi.org/10.1097/SLA.0000000000000585.
VIRTUE Registry Investigators. Mid-term outcomes and aortic remodelling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE registry. Eur J Vasc Endovasc Surg. 2014;48:363–71. https://doi.org/10.1016/j.ejvs.2014.05.007.
Zipfel B, Zaefferer P, Riambau V, et al. Worldwide results from the RESTORE II on elective endografting of thoracic aneurysms and dissections. J Vasc Surg. 2016;63:1466–75. https://doi.org/10.1016/j.jvs.2015.12.032.
Zipfel B, Czerny M, Funovics M, et al. RESTORE Investigators. endovascular treatment of patients with types A and B thoracic aortic dissection using relay thoracic stent-grafts: results from the restore patient registry. J Endovasc Ther. 2011;18:131–43.
Spanos K, Kölbel T. Device profile of the Zenith dissection endovascular system for aortic dissection. Expert Rev Med Devices. 2019;16:541–8. https://doi.org/10.1080/17434440.2019.1627198.
Cambria RP, Crawford RS, Cho JS, et al. GORE TAG Investigators. a multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50(1255–64):e1–4. https://doi.org/10.1016/j.jvs.2009.07.104.
Rousseau H, Revel-Mouroz P, Saint Lebes B, et al. Single aortic branch device: the Mona LSA experience. J Cardiovasc Surg (Torino). 2019;60:81–90. https://doi.org/10.23736/S0021-9509.18.10665-3.
Patel HJ, Dake MD, Bavaria JE, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the Gore thoracic branch endoprosthesis. Ann Thorac Surg. 2016;102:1190–8.
Alsafi A, Bicknell CD, Rudarakanchana N, et al. Endovascular treatment of thoracic aortic aneurysms with a short proximal landing zone using scalloped endografts. J Vasc Surg. 2014;60:1499–506. https://doi.org/10.1016/j.jvs.2014.08.062.
Chassin-Trubert L, Mandelli M, Ozdemir BA, et al. Midterm follow-up of fenestrated and scalloped physician-modified endovascular grafts for zone 2 TEVAR. J Endovasc Ther. 2019;24:1526602819881128. https://doi.org/10.1177/1526602819881128.
Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. 2019;55:133–62. https://doi.org/10.1093/ejcts/ezy313.
Bosiers MJ, Donas KP, Mangialardi N, et al. European multicenter registry for the performance of the chimney/snorkel technique in the treatment of aortic arch-pathologic conditions. Ann Thorac Surg. 2016;101:2224–30.
Mangialardi N, Serrao E, Kasemi H, et al. Chimney technique for aortic arch pathologies: an 11-year singlecenter experience. J Endovasc Ther. 2014;21:312–23.
Liu F, Ge YY, Guo W, et al. Preoperative thoracic false lumen branches are predictors of aortic enlargement after stent grafting for DeBakey IIIb aortic dissection. J Thorac Cardiovasc Surg. 2018;155(21–9):e3. https://doi.org/10.1016/j.jtcvs.2017.09.010.
Watanabe Y, Shimamura K, Yoshida T, et al. Aortic remodeling as a prognostic factor for late aortic events after thoracic endovascular aortic repair in type B aortic dissection with patent false lumen. J Endovasc Ther. 2014;21:517–25. https://doi.org/10.1583/13-4646R.1.
Hughes GC. Stent graft-induced new entry tear (SINE): Intentional and NOT. J Thorac Cardiovasc Surg. 2019;157(1):101–106.e3. https://doi.org/10.1016/j.jtcvs.2018.10.060.
Kitagawa A, Greenberg RK, Eagleton MJ, et al. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection with thoracoabdominal aneurysms. J Vasc Surg. 2013;58:625–34. https://doi.org/10.1016/j.jvs.2013.01.049.
Oikonomou K, Kasprzak P, Katsargyris A, et al. Mid-term results of fenestrated/branched stent grafting to treat post-dissection thoraco-abdominal aneurysms. Eur J Vasc Endovasc Surg. 2019;57:102–9. https://doi.org/10.1016/j.ejvs.2018.07.032.
Law Y, Tsilimparis N, Rohlffs F, et al. Fenestrated or branched endovascular aortic repair for postdissection thoracoabdominal aortic aneurysm. J Vasc Surg. 2019;70:404–12. https://doi.org/10.1016/j.jvs.2018.10.117.
Bertoglio L, Rinaldi E, Melissano G, Chiesa R. The PETTICOAT concept for endovascular treatment of type B aortic dissection. J Cardiovasc Surg (Torino). 2019;60:91–9. https://doi.org/10.23736/S0021-9509.17.09744-0.
Lombardi JV, Gleason TG, Panneton JM, et al. STABLE II clinical trial on endovascular treatment of acute, complicated type B aortic dissection with a composite device design. J Vasc Surg. 2020;71(1077–87):e2. https://doi.org/10.1016/j.jvs.2019.06.189.
Lombardi JV, Cambria RP, Nienaber CA, et al. Aortic remodeling after endovascular treatment of complicated type B aortic dissection with the use of a composite device design. J Vasc Surg. 2014;59:1544–54. https://doi.org/10.1016/j.jvs.2013.12.
Kazimierczak A, Rynio P, Jędrzejczak T, et al. Expanded Petticoat technique to promote the reduction of contrasted false lumen volume in patients with chronic type B aortic dissection. J Vasc Surg. 2019;S0741–5214(19):30343. https://doi.org/10.1016/j.jvs.2019.01.073.
Ibrahim W, Spanos K, Gussmann A, et al. Early and midterm outcome of multilayer flow modulator stent for complex aortic aneurysm treatment in Germany. J Vasc Surg. 2018;68:956–64. https://doi.org/10.1016/j.jvs.2018.01.037.
Sultan S, Kavanagh EP, Stefanov F, et al. Global MFM Collaborators. endovascular management of chronic symptomatic aortic dissection with the streamliner multilayer flow modulator: twelve-month outcomes from the global registry. J Vasc Surg. 2017;65:940–50. https://doi.org/10.1016/j.jvs.2016.09.059.
Kamman AV, Jonker FHW, Sechtem U, et al. IRAD investigators. predictors of stable aortic dimensions in medically managed acute aortic syndromes. Ann Vasc Surg. 2017;42:143–9.
Li D, Ye L, He Y, et al. False lumen status in patients with acute aortic dissection: a systematic review and meta-analysis. J Am Heart Assoc. 2016;10:5.
Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357:349–59.
Loubert MC, van der Hulst VP, De Vries C, et al. How to exclude the dilated false lumen in patients after a type B aortic dissection? The cork in the bottleneck. J Endovasc Ther. 2003;10:244–8. https://doi.org/10.1177/152660280301000213.
Spanos K, Kölbel T, Rohlffs F, et al. Intentional targeted false lumen occlusion after aortic dissection: a systematic review of the literature. Ann Vasc Surg. 2019;56:317–29. https://doi.org/10.1016/j.avsg.2018.08.086.
Kölbel T, Lohrenz C, Kieback A, et al. Distal false lumen occlusion in aortic dissection with a homemade extra-large vascular plug: the candy-plug technique. J Endovasc Ther. 2013;20:484–9. https://doi.org/10.1583/13-4318.1.
Kölbel T, Carpenter SW, Lohrenz C, et al. Addressing persistent false lumen flow in chronic aortic dissection: the knickerbocker technique. J Endovasc Ther. 2014;21:117–22. https://doi.org/10.1583/13-4463MR-R.1.
Rohlffs F, Tsilimparis N, Fiorucci B, et al. The candy-plug technique: technical aspects and early results of a new endovascular method for false lumen occlusion in chronic aortic dissection. J Endovasc Ther. 2017;24:549–55. https://doi.org/10.1177/1526602817709252.
Eleshra A, Kölbel T, Tsilimparis N, et al. Candy-plug generation ii for false lumen occlusion in chronic aortic dissection: feasibility and early results. J Endovasc Ther. 2019;3:1526602819871613. https://doi.org/10.1177/1526602819871613.
Pellenc Q, Roussel A, De Blic R, et al. False lumen embolization in chronic aortic dissection promotes thoracic aortic remodeling at midterm follow-up. J Vasc Surg. 2019;70:710–7. https://doi.org/10.1016/j.jvs.2018.11.038.
Crawford ES. The diagnosis and management of aortic dissection. JAMA. 1990;264:2537–41.
Fiorucci B, Kölbel T, Rohlffs F, et al. The role of thoracic endovascular repair in elective, symptomatic and ruptured thoracic aortic diseases. Eur J Cardiothorac Surg. 2019;56:197–203. https://doi.org/10.1093/ejcts/ezy482.
Nakamura K, Uchida T, Sho R, et al. Analysis of risk factors for aortic enlargement in patients with chronic type B aortic dissection. Ann Vasc Dis. 2018;11:490–5. https://doi.org/10.3400/avd.oa.18-00115.
Sze DY, van den Bosch MA, Dake MD, et al. Factors portending endoleak formation after thoracic aortic stent-graft repair of complicated aortic dissection. Circ Cardiovasc Interv. 2009;2:105–12.
Sharafuddin MJ, Reece TB, Papia G, et al. Proposed classification of endoleaks after endovascular treatment of stanford type-B aortic dissections. Vascular. 2019;8:1708538119847394. https://doi.org/10.1177/1708538119847394.
Waterford SD, Chou D, Bombien R, et al. Left subclavian arterial coverage and stroke during thoracic aortic endografting: a systematic review. Ann Thorac Surg. 2016;101:381–9. https://doi.org/10.1016/j.athoracsur.2015.05.138.
Chen X, Wang J, Premaratne S, et al. Meta-analysis of the outcomes of revascularization after intentional coverage of the left subclavian artery for thoracic endovascular aortic repair. J Vasc Surg. 2019;70:1330–40. https://doi.org/10.1016/j.jvs.2019.03.022.
Konstantinou N, Debus ES, Vermeulen CFW, et al. Cervical debranching in the endovascular era: a single centre experience. Eur J Vasc Endovasc Surg. 2019;58:34–40. https://doi.org/10.1016/j.ejvs.2018.12.010.
Kölbel T, Rohlffs F, Wipper S, et al. Carbon dioxide flushing technique to prevent cerebral arterial air embolism and stroke during TEVAR. J Endovasc Ther. 2016;23:393–5. https://doi.org/10.1177/1526602816633705.
Rohlffs F, Trepte C, Ivancev K, et al. Air embolism during TEVAR: liquid perfluorocarbon absorbs carbon dioxide in a combined flushing technique and decreases the amount of gas released from thoracic stent-grafts during deployment in an experimental setting. J Endovasc Ther. 2019;26:76–80. https://doi.org/10.1177/1526602818819501.
Li HL, Wu S, Chan YC, et al. Early and mid-term mortality and morbidity of contemporary international endovascular treatment for type B aortic dissection—a systematic review and meta-analysis. Int J Cardiol. 2020;301:56–61. https://doi.org/10.1016/j.ijcard.2019.09.071.
Xue Y, Ge Y, Ge X, et al. Association between extent of stent-graft coverage and thoracic aortic remodeling after endovascular repair of type B aortic dissection. J Endovasc Ther. 2020;27:211–20.
Canaud L, Gandet T, Sfeir J, et al. Risk factors for distal stent graft induced new entry tear after endovascular repair of thoracicaortic dissection. J Vasc Surg. 2019;69:1610–4. https://doi.org/10.1016/j.jvs.2018.07.086.
D'cruz RT, Syn N, Wee I, et al. Singapore vascular surgical collaborative (SingVaSC). risk factors for distal stent graft-induced new entry in type B aortic dissections: systematic review and meta-analysis. J Vasc Surg. 2019;70:1682–93.
Lortz J, Leinburger F, Tsagakis K, et al. Distal stent graft induced new entry: risk factors in acute and chronic type B aortic dissections. Eur J Vasc Endovasc Surg. 2019;S1078–5884(19):30292–8. https://doi.org/10.1016/j.ejvs.2019.04.015.
Funding
Open access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tilo Kölbel has intellectual property with Cook Medical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Spanos, K., Kölbel, T. Role of Endoluminal Techniques in the Management of Chronic Type B Aortic Dissection. Cardiovasc Intervent Radiol 43, 1808–1820 (2020). https://doi.org/10.1007/s00270-020-02566-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02566-7