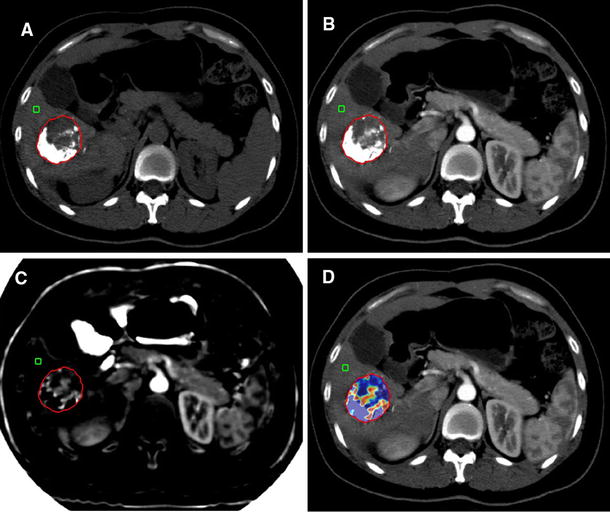
Abstract
Purpose
To retrospectively compare early response to yttrium-90 radioembolization (Y90) according to volumetric iodine uptake (VIU) changes, Response Evaluation Criteria In Solid Tumor 1.1 (RECIST 1.1) and modified RECIST (mRECIST) in patients with intermediate-advanced hepatocellular carcinoma (HCC) and to explore their association with survival.
Materials and Methods
Twenty-four patients treated with Y90 and evaluated with dual-energy computed tomography before and 6 weeks after treatment were included. VIU was measured on late arterial phase spectral images; 6-week VIU response was defined as: complete response (CR, absence of enhancing tumor), partial response (PR, ≥ 15% VIU reduction), progressive disease (PD, ≥ 10% VIU increase) and stable disease (criteria of CR/PR/PD not met). RECIST 1.1 and mRECIST were evaluated at 6 weeks and 6 months. Responders included CR and PR. Overall survival (OS) was evaluated by Kaplan–Meier analysis and compared by Cox regression analysis.
Results
High intraobserver and interobserver agreements were observed in VIU measurements (k > 0.98). VIU identified a higher number of responders (18 patients, 75%), compared to RECIST 1.1 (12.5% at 6 weeks and 23.8% at 6 months) and mRECIST (29.2% at 6 weeks and 61.9% at 6 months). There was no significant correlation between OS and RECIST 1.1 (P = 0.45 at 6 weeks; P = 0.21 at 6 months) or mRECIST (P = 0.38 at 6 weeks; P = 0.79 at 6 months); median OS was significantly higher in VIU responders (17.2 months) compared to non-responders (7.4 months) (P = 0.0022; HR 8.85; 95% CI 1.29–88.1).
Conclusion
VIU is highly reproducible; as opposite to mRECIST and RECIST 1.1, early VIU response correlates with OS after Y90 in intermediate-advanced HCC patients.



Similar content being viewed by others
References
Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–16.
Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–18.
Jung ES, Kim JH, Yoon EL, et al. Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Hepatol. 2013;58(6):1181–7.
Vincenzi B, Di Maio M, Silletta M, et al. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS ONE. 2015;10(7):0133488.
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
Atassi B, Bangash AK, Bahrani A, et al. Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics. 2008;28:81–99.
Ibrahim SM, Nikolaidis P, Miller FH, et al. Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging. 2009;34:566–81.
Vouche M, Kulik L, Atassi R, et al. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: imaging analysis from a prospective randomized trial of Y90 ± sorafenib. Hepatology. 2013;58:1655–66.
Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, et al. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology. 2015;62:1111–21.
Galizia MS, Töre HG, Chalian H, et al. MDCT necrosis quantification in the assessment of hepatocellular carcinoma response to yttrium 90 radioembolization therapy: comparison of two-dimensional and volumetric techniques. Acad Radiol. 2012;19:48–54.
Duke E, Deng J, Ibrahim SM, et al. Agreement between competing imaging measures of response of hepatocellular carcinoma to yttrium-90 radioembolization. J Vasc Interv Radiol. 2010;21:515–21.
Chapiro J, Lin M, Duran R, et al. Assessing tumor response after loco-regional liver cancer therapies: the role of 3D MRI. Expert Rev Anticancer Ther. 2015;15:199–205.
Reiner CS, Gordic S, Puippe G, et al. Histogram analysis of CT perfusion of hepatocellular carcinoma for predicting response to transarterial radioembolization: value of tumor heterogeneity assessment. Cardiovasc Interv Radiol. 2016;39:400–8.
Kokabi N, Camacho JC, Xing M, et al. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39:969–78.
Weng Z, Ertle J, Zheng S, et al. Choi criteria are superior in evaluating tumor response in patients treated with transarterial radioembolization for hepatocellular carcinoma. Oncol Lett. 2013;6:1707–12.
Vouche M, Salem R, Lewandowski RJ, et al. Can volumetric ADC measurement help predict response to Y90 radioembolization in HCC? Abdom Imaging. 2015;40:1471–80.
Tacher V, Lin M, Duran R, et al. Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology. 2016;278:275–84.
Bonekamp S, Halappa VG, Geschwind JF, et al. Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part II. Response stratification using volumetric functional criteria after intraarterial therapy. Radiology. 2013;268:431–9.
Rathmann N, Budjan J, Mari F, et al. Semiautomatic whole-lesion apparent diffusion coefficient assessment for early prediction of liver tumor response to radioembolization. Anticancer Res. 2016;36:2961–6.
Zhu X, Sobhani F, Xu C, et al. Quantitative volumetric functional MR imaging: an imaging biomarker of early treatment response in hypo-vascular liver metastasis patients after yttrium-90 transarterial radioembolization. Abdom Radiol (NY). 2016;41:1495–504.
Bonekamp D, Bonekamp S, Halappa VG, et al. Interobserver agreement of semi-automated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol. 2014;83:487–96.
Gordic S, Puippe GD, Krauss B, et al. Correlation between dual-energy and perfusion CT in patients with hepatocellular carcinoma. Radiology. 2016;280:78–87.
Reiner CS, Morsbach F, Sah BR, et al. Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vasc Interv Radiol. 2014;25:747–59.
Dai X, Schlemmer HP, Schmidt B, et al. Quantitative therapy response assessment by volumetric iodine-uptake measurement: initial experience in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur J Radiol. 2013;82:327–34.
Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073–80.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Forner A, Ayuso C, Varela M, et al. Locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–23.
Keppke AL, Salem R, Reddy D, et al. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol. 2007;188:768–75.
Riaz A, Gabr A, Abouchaleh N, et al. Radioembolization for hepatocellular carcinoma: statistical confirmation of improved survival in responders by landmark analyses. Hepatology. 2018;67:873–83.
Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–37.
Sangro B, Carpanese L, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–78.
De la Torre M, Buades-Mateu J, de la Rosa PA, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or Sorafenib. Liver Int. 2016;36:1206–12.
Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507.
Rhee TK, Naik NK, Deng J, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008;19:1180–6.
Agrawal MD, Pinho DF, Kulkarni NM, et al. Oncologic applications of dual-energy CT in the abdomen. Radiographics. 2014;34:589–612.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Irene Bargellini received honoraria from GE Healthcare, Biocompatibles UK LTD, Sirtex, Bayer Spa; Laura Crocetti received honoraria from GE Healthcare; the remaining authors have no conflict of interests to declare.
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed Consent
For this type of study, informed consent was waived by the Local Ethical Committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bargellini, I., Crocetti, L., Turini, F.M. et al. Response Assessment by Volumetric Iodine Uptake Measurement: Preliminary Experience in Patients with Intermediate-Advanced Hepatocellular Carcinoma Treated with Yttrium-90 Radioembolization. Cardiovasc Intervent Radiol 41, 1373–1383 (2018). https://doi.org/10.1007/s00270-018-1962-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1962-8