Abstract
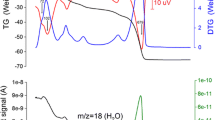
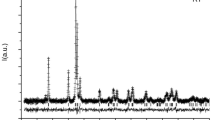
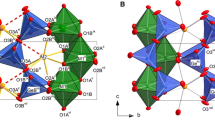
The crystal structure of ferrinatrite, Na3[Fe(SO4)3]·3H2O, was refined based on a new single-crystal X-ray diffraction experiment on a sample from the type locality Sierra Gorda, Chile. The data allowed H to be successfully located and the H-bonding system to be defined. Infrared and Raman spectra are presented and discussed for this compound on the basis of the crystal structure. The Oacceptor···H–Odonor bond distances determined from the structure refinement agree well with the geometric correlation obtained from spectroscopic data. The thermal stability and dehydration process of ferrinatrite was investigated by in situ high temperature (HT) synchrotron X-ray powder diffraction, Raman and Fourier transform infrared spectroscopies.











Similar content being viewed by others
References
Adler HH, Kerr PF (1965) Variations in infrared spectra, molecular symmetry and site symmetry of sulfate minerals. Am Miner 50:132–147
Balic-Zunic T, Garavelli A, Acquafredda P, Leonardsen E, Jakobsson SP (2009) Eldfellite, NaFe(SO4)2, a new fumarolic mineral from Eldfell volcano. Iceland Mineral Mag 73:51–57
Bandy MC (1938) Mineralogy of three sulphate deposits of Northern Chile. Am Miner 23:669–760
Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ (2003) Crystals version 12: Software for guided crystal structure analysis. J Appl Cryst 36:1487
Blessing RH (1995) An empirical correction for absorption anisotropy. Acta Cryst A 51:33–38
Boghosian S, Fehrmann R, Nielsen K (1994) Synthesis and crystal structure of Na3V(SO4)3. Spectroscopic characterization of Na3V(SO4)3 and NaV(SO4)2. Acta Chem Scand 48:724–731
Boudjada A, Guitel JC (1981) Structure crystalline d’un orthoarséniate acide de fer(III) pentahydraté: Fe(H2AsO4)3·5H2O. Acta Cryst B 37:1402–1405
Brese NE, O’Keeffe M (1991) Bond-valence parameters for solids. Acta Cryst B 47:192–197
Brown ID, Altermatt D (1985) Bond valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Cryst B 41:244–247
Bruker B (2008) APEX2, SAINT and TWINABS. AXS Inc., Madison
Césbron F (1964) Contribution à la Minéralogie des sulphates de fer hydrates. B Soc Fr Mineral Cr 87:125–143
Della Ventura G, Gatta D, Redhammer GJ, Bellatreccia F, Loose A, Parodi GC (2009) Single-crystal polarized FTIR spectroscopy and neutron diffraction refinement of cancrinite. Phys Chem Minerals 36:193–206
Della Ventura G, Ventruti G, Bellatreccia F, Scordari F, Cestelli Guidi M (2013) FTIR transmission spectroscopy of sideronatrite, a sodium-iron hydrous sulphate. Mineral Mag 77:499–507
Demartin F, Castellano C, Gramaccioli CM, Campostrini I (2010a) Aluminum-for-iron substitution, hydrogen bonding, and a novel structure-type in coquimbite-like minerals. Can Mineral 48:323–333
Demartin F, Gramaccioli CM, Campostrini I (2010b) Pyracmonite, (NH4)3Fe(SO4)3, a new ammonium iron sulfate from La Fossa crater, Vulcano, Aeolian islands, Italy. Can Mineral 48:307–313
Fang JH, Robinson PD (1970) Crystal structures and mineral chemistry of hydrated ferric sulfates. I. The crystal structure of coquimbite. Am Miner 55:1534–1540
Hammersley AP (1998) Fit2D: V9.129 Reference Manual Version 3.1. Internal Report ESRF—98 – HA01
Hawthorne FC, Krivovichev SV, Burns PC (2000) The crystal chemistry of sulfate minerals. In: Alpers CN, Jambor JL, Nordstrom BK (eds) Sulfate minerals: crystallography, geochemistry, and environmental significance, Reviews in Mineralogy and Geochemistry, vol 40. Mineralogical Society of America and the Geochemical Society, Washington, pp 1–112
Hoppe R (1979) Effective coordination numbers (ECoN) and mean fictive ionic radii (MEFIR)[1, 2]*. Z Kristallogr 150:23–52
Jambor JL, Nordstrom DK, Alpers CN (2000) Metal-sulfate salts from sulfide mineral oxidation. In: Alpers CN, Jambor JL, Nordstrom DK (eds) Sulfate minerals: crystallography, geochemistry, and environmental significance, Reviews in Mineralogy and Geochemistry, vol 40. Mineralogical Society of America and the Geochemical Society, Washington, pp 305–350
Kampf AR, Mills SJ, Nash BP, Dini M, Molina Donoso AA (2017) Currierite, Na4Ca3MgAl4(AsO3OH)12·9H2O, a new acid arsenate with ferrinatrite-like heteropolyhedral chains from the Torrecillas mine, Iquique Province, Chile. Mineral Mag 81:1141–1149
Libowitzky E (1999) Correlation of O-H stretching frequencies and O–H⋯O hydrogen bond lengths in minerals. Monatsh Chem 130:1047–1059
Mackintosh JB (1889) Notes on some native iron sulphates from Chili. Am J Sci 38:242–245
Majzlan J, Navrotsky A, McCleskey RB, Alpers CN (2006) Thermodynamic properties and crystal structure refinement of ferricopiapite, coquimbite, rhomboclase, and Fe2(SO4)3(H2O)5. Eur J Mineral 18:175–186
Matýsek D, Jirásek J, Osovský M, Skupien P (2014) Minerals formed by the weathering of sulfides in mines of the Czech part of the Upper Silesian Basin. Mineral Mag 78:1265–1286
Murray J, Kirschbaum A, Dold B, Guimaraes EM, Miner EP (2014) Jarosite versus soluble iron-sulfate formation and their role in acid mine drainage formation at the Pan de Azúcar Mine Tailings (Zn–Pb–Ag), NW Argentina. Mineral-Basel 4:477–502
Nakamoto K (1997) Infrared and raman spectra of inorganic and coordination compounds, 5th edn. Wiley, New York
Nespolo M, Ferraris G, Ivaldi G, Hoppe R (2001) Charge distribution as a tool to investigate structural details. II. Extension to hydrogen bonds, distorted and hetero-ligand polyhedra. Acta Cryst B 57:652–664
Palache C, Berman H, Frondel C (1951) Dana’s system of mineralogy. John, New York
Perchiazzi N, Ondruš P, Skála R (2004) Ab initio X-ray powder structure determination of parascorodite, Fe(H2O)2AsO4. Eur J Mineral 16:1003–1007
Plaisier JR, Nodari L, Gigli L, Rebollo San Miguel EP, Bertoncello R, Lausi A (2017) The X-ray diffraction beamline MCX at Elettra: a case study of non-destructive analysis on stained glass. Acta Imeko 6:71–75
Riello P, Lausi A, Macleod J, Plaisier JR, Zerauschek G, Fornasiero P (2013) In situ reaction furnace for real-time XRD studies. J Synchrotron Radiat 20:194–196
Robinson PD, Fang JH (1971) Crystal structures and mineral chemistry of hydrated ferric sulphates: II. The crystal structure of paracoquimbite. Am Miner 56:1567–1572
Ross SD (1974) Sulphates and other oxy-anions of group VI. In: Farmer VC (ed) The Infrared Spectra of Minerals, Mineralogical Society of Great Britain and Ireland, pp 423–444
Rouchon V, Badet H, Belhadj O, Bonnerot O, Lavédrine B, Michard J-G, Miska S (2012) Raman and FTIR spectroscopy applied to the conservation report of paleontological collections: identification of Raman and FTIR signatures of several iron sulfate species such as ferrinatrite and sideronatrite. J Raman Spectrosc 43:1265–1274
Scordari F (1977) The crystal structure of ferrinatrite, Na3(H2O)3[Fe(SO4)3] and its relationship to Maus’s salt, (H3O)2K2{K0.5(H2O)0.5}6[Fe3O(H2O)3(SO4)6](OH)2. Mineral Mag 41:375–383
Scordari F, Ventruti G (2009) Sideronatrite Na2[Fe(SO4)2OH] ·3H2O: Crystal structure of the orthorhombic polytype and OD character analysis. Am Miner 94:1679–1686
Scordari F, Ventruti G, Gualtieri AF, Lausi A (2011) Crystal structure of Na3Fe(SO4)3: a high temperature product (~ 400 °C) of sideronatrite Na2[Fe(SO4)2OH·3H2O]. Am Miner 96:1107–1111
Sheldrick GM (2003) XPREP version 6.14. Bruker-Nonius Inc., Madison
Ventruti G, Stasi F, Scordari F (2010) Metasideronatrite: crystal structure and its relation with sideronatrite. Am Miner 95:329–334
Ventruti G, Scordari F, Della Ventura G, Bellatreccia F, Gualtieri AF, Lausi A (2013) The thermal stability of sideronatrite and its decomposition products in the system Na2O–Fe2O3–SO2–H2O. Phys Chem Minerals 40:659–670
Ventruti G, Della Ventura G, Bellatreccia F, Lacalamita M, Schingaro E (2016) Hydrogen bond system and vibrational spectroscopy of the iron sulfate fibroferrite, Fe(OH)SO4·5H2O. Eur J Mineral 28:943–952
Woińska M, Grabowsky S, Dominiak PM, Woźniak K, Jayatilaka D (2016) Hydrogen atoms can be located accurately and precisely by X-ray crystallography. Sci Adv 2:e1600192
Yang Z, Giester G, Ding K, Li H (2015) Crystal structure of sideronatrite-2M, Na2Fe(SO4)2(OH)(H2O)3, a new polytype from Xitieshan lead-zinc deposit, Qinghai province, China. Eur J Mineral 27:427–432
Acknowledgements
We thank Diamond Light Source for access to MIRIAM beamline B22 (proposal number SM17483). We thank two reviewers, S. Mills and an anonymous reviewer for their constructive comments which allowed us to improve the clarity of the manuscript. The research leading to the results presented here has received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under the grant agreement n◦ 226716. The micro-Raman laboratory at University of Bari “Aldo Moro”, was funded by Potenziamento Strutturale PONa3_00369 “Laboratorio per lo Sviluppo Integrato delle Scienze e delle TEcnologie dei Materiali Avanzati e per dispositivi innovativi (SISTEMA)”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ventruti, G., Ventura, G.D., Lacalamita, M. et al. Crystal-chemistry and vibrational spectroscopy of ferrinatrite, Na3[Fe(SO4)3]·3H2O, and its high-temperature decomposition. Phys Chem Minerals 46, 119–131 (2019). https://doi.org/10.1007/s00269-018-0991-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-018-0991-9