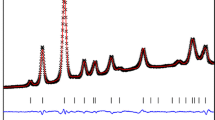
Abstract
The P–T phase stability field, the thermoelastic behavior and the P-induced deformation mechanisms at the atomic scale of pargasite crystals, from the “phlogopite peridotite unit” of the Finero mafic–ultramafic complex (Ivrea-Verbano Formation, Italy), have been investigated by a series of in situ experiments: (a) at high pressure (up to 20.1 GPa), by single-crystal synchrotron X-ray diffraction with a diamond anvil cell, (b) at high temperature (up to 823 K), by powder synchrotron X-ray diffraction using a hot air blower device, and (c) at simultaneous HP–HT conditions, by single-crystal synchrotron X-ray diffraction with a resistive-heated diamond anvil cell (P max = 16.5 GPa, T max = 1200 K). No phase transition has been observed within the P–T range investigated. At ambient T, the refined compressional parameters, calculated by fitting a second-order Birch–Murnaghan Equation of State (BM-EoS), are: V 0 = 915.2(8) Å3 and K P0,T0 = 95(2) GPa (β P0,T0 = 0.0121(2) GPa−1) for the unit-cell volume; a 0 = 9.909(4) Å and K(a) P0,T0 = 76(2) GPa for the a-axis; b 0 = 18.066(7) Å and K(b) P0,T0 = 111(2) GPa for the b-axis; c 0 = 5.299(5) Å and K(c) P0,T0 = 122(12) GPa for the c-axis [K(c) P0,T0 ~ K(b) P0,T0 > K(a) P0,T0]. The high-pressure structure refinements (at ambient T) show a moderate contraction of the TO4 double chain and a decrease of its bending in response to the hydrostatic compression, along with a pronounced compressibility of the A- and M(4)-polyhedra [K P0, T0(A) = 38(2) GPa, K P0, T0(M4) = 79(5) GPa] if compared to the M(1)-, M(2)-, M(3)-octahedra [K P0, T0(M1,2,3) ≤ 120 GPa] and to the rigid tetrahedra [K P0, T0(T1,T2) ~ 300 GPa]. The thermal behavior, at ambient pressure up to 823 K, was modelled with Berman’s formalism, which gives: V 0 = 909.1(2) Å3, α0 = 2.7(2)·10−5 K−1 and α1 = 1.4(6)·10−9 K−2 [with α0(a) = 0.47(6)·10−5 K−1, α0(b) = 1.07(4)·10−5 K−1, and α0(c) = 0.97(7)·10−5 K−1]. The petrological implications for the experimental findings of this study are discussed.








Similar content being viewed by others
References
Agilent Technologies (2011) Xcalibur CCD system, CrysAlisPro Software system, Version 1.171.35.XX. Agilent Technologies, Oxford
Angel RJ (2000) High-temperature and high-pressure crystal chemistry. Rev Miner Geochem 41:35–60
Angel RJ, Bujak M, Zhao J, Gatta GD, Jacobsen SD (2007) Effective hydrostatic limits of pressure media for high-pressure crystallographic studies. J Appl Crystallogr 40:26–32
Angel RJ, Alvaro M, Gonzalez-Platas J (2014) EosFit7c and a Fortran module (library) for equation of state calculations. Z Kristallogr 229:405–419
Berman RG (1988) Internally-consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2–H2O-CO2. J Pet 29:445–522
Boffa Ballaran T, Angel RJ, Carpenter MA (2000) High-pressure transformation behaviour of the cummingtonite-grunerite solid solution. Eur J Miner 12:1195–1213
Bose K, Ganguly J (1994) Thermogravimetric study of the dehydration kinetics of talc Am Mineral 79:692–699
Cámara F, Oberti R, Iezzi G, Della Ventura G (2003) The P21/m ↔ C2/m phase transition in synthetic amphibole Na(NaMg)Mg5Si8O22(OH)2: thermodynamic and crystal-chemical evaluation. Phys Chem Miner 30:570–581
Cámara F, Oberti R, Casati N (2007) The P21/m ↔ C2/m phase transition in amphiboles: new data on synthetic Na(NaMg)Mg5Si8O22F2 and the role of differential polyhedral expansion. Z Kristallogr 223:148–159
Cameron M, Sueno S, Papike JJ, Prewitt CT (1983) High temperature crystal chemistry of K and Na fluor-richterites. Am Mineral 68:924–943
Cawthorn RG (1975) The amphibole peridotite–metagabbro complex, Finero, northern Italy. J Geol 83:437–454
Coltorti M, Siena F (1984) Mantle tectonite and fractionate peridotite at Finero (Italian Western Alps). Neues Jahrb Miner Abh 149:225–244
Comodi P, Mellini M, Ungaretti L, Zanazzi PF (1991) Compressibility and high pressure structure refinement of tremolite, pargasite, and glaucophane. Eur J Miner 3:485–500
Comodi P, Boffa Ballaran T, Zanazzi PF, Capalbo C, Zanetti A, Nazzareni S (2010) The effect of oxo-component on the high-pressure behavior of amphiboles. Am Mineral 95:1042–1051
Fei Y, Ricolleau A, Frank M, Mibe K, Shen G, Prakapenka V (2007) Toward an internally consistent pressure scale. Proc Natl Acad Sci USA 104:9182–9186
Foley F, Tiepolo M, Vannucci R (2002) Growth of early continental crust controlled by melting of amphibolite in subduction zones. Nature 417:837–840
Forneris JF, Holloway JR (2003) Phase equilibria in subducting basaltic crust: implications for H2O release from the slab. Earth Planet Sci Lett 214:187–201
Fumagalli P, Poli S (2005) Experimentally determined phase relations in hydrous peridotites to 6.5 GPa and their consequences on the dynamics of subduction zones. J Pet 46:555–578
Gatta GD, McIntyre GJ, Oberti R, Hawthorne FC (2017) Order of [6]Ti4+ in a Ti-rich calcium amphibole from Kaersut, Greenland: a combined X-ray and neutron diffraction study. Phys Chem Miner 44:83–94
Gill J (1981) Orogenic andesites and plate tectonics. Springer, New York, p 390
Green DH, Wallace ME (1988) Mantle metasomatism by ephemeral carbonatite melt. Nature 336:459–462
Hawthorne FC (1981) Amphiboles and other hydrous pyriboles-mineralogy. Rev Miner 9A:1–102
Hawthorne FC, Oberti R (2007) Amphiboles: crystal chemistry. Rev Miner Geochem 67:1–54
Hawthorne FC, Oberti R, Sardone N (1996) Sodium at the A site in clinoamphiboles: the effects of composition on patterns of order. Can Miner 34:577–593
Iezzi G, Tribaudino M, Della Ventura G, Nestola F, Bellatreccia F (2005a) High-T phase transition of synthetic ANaB(LiMg)CMg5Si8O22(OH)2 amphibole: an X-ray synchrotron powder diffraction and FTIR spectroscopic study. Phys Chem Miner 32:515–523
Iezzi G, Gatta GD, Kockelmann W, Della Ventura G, Rinaldi R, Schäfer W, Piccinini M, Gaillard F (2005b) Low-T neutron powder-diffraction and synchrotron-radiation IR study of synthetic amphibole Na(NaMg)Mg5Si8O22(OH)2. Am Mineral 90:695–700
Iezzi G, Tribaudino M, Della Ventura G, Margiolaki I (2011) The high temperature P21/m → C2/m phase transitions in synthetic amphiboles along the richterite-(BMg)-richterite join. Am Mineral 96:353–363
Ionov DA, Hofmann AW (1995) Nb-Ta-rich mantle amphiboles and micas: implications for subduction-related metasomatic trace element fractionations. Earth Planet Sci Lett 131:341–356
Ionov DA, Bodinjer JL, Mukasa SB, Zanetti A (2002) Mechanisms and sources of mantle metasomatism: major and trace element compositions of peridotite xenoliths from Spitsbergen in the context of numerical modelling. J Pet 43:2219–2259
Jenkins DM, Corona JC (2006) Molar volume and thermal expansion of glaucophane. Phys Chem Miner 33:356–362
Jenkins DM, Corona JC, Bassett WA, Mibe K, Wang ZW (2010) Compressibility of synthetic glaucophane. Phys Chem Miner 37:219–226
Klotz S, Chervin JC, Munsch P, Le Marchand G (2009) Hydrostatic limits of 11 pressure transmitting media. J Phys D Appl Phys 42:075413. doi:10.1088/0022-3727/42/7/075413 (7 pp)
Larson AC, Von Dreele RB (1994) GSAS Generalized structure analysis system. Los Alamos National Laboratory Report LAUR 86–748
Le Bail A, Duroy H, Fourquet JL (1988) Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat Res Bull 23:447–452
Mandler BE, Grove TL (2016) Controls on the stability and composition of amphibole in the Earth’s mantle. Contrib Miner Pet 171:68–87
Mao HK, Xu J, Bell PM (1986) Calibration of the ruby pressure gauge to 800-kbar under quasi-hydrostatic conditions. J Geophys Res 91:4673–4676
Nestola F, Pasqual D, Welch MD, Oberti R (2012) The effects of composition upon the high-pressure behaviour of amphiboles: compression of gedrite to 7 GPa and a comparison with anthophyllite and proto-amphibole. Miner Mag 76:987–995
Niida K, Green DH (1999) Stability and chemical composition of pargasitic amphibole in MORB pyrolite under upper mantle conditions. Contr Miner Pet 135:18–40
Papike JJ, Ross M, Clark JR (1969) Crystal chemical characterization of clinoamphiboles based on five new structure refinements. Miner Soc Am Spec Pap 2:117–136
Petříček V, Dušek M, Palatinus L (2014) Crystallographic computing system JANA2006: general features. Z Kristallogr 229:345–352
Poli S, Schmidt MW (1995) Water transport and release in subduction zones: experimental constraints on basaltic and andesitic systems. J Geophys Res 100:22299–22314
Rebuffi L, Plaisier JR, Abdellatief M, Lausi A, Scardi P (2014) MCX: a synchrotron radiation beamline for X-ray diffraction line profile analysis. Z Anorg Allg Chem 640:3100–3106
Reece JJ, Redfern SAT, Welch MD, Henderson CMB (2000) Mn-Mg disordering in cummingtonite: a high temperature neutron powder diffraction study. Miner Mag 64:255–266
Reece JJ, Redfern SAT, Welch MD, Henderson CMB, McCammon CA (2002) Temperature-dependent Fe2+-Mn2+ order-disorder behaviour in amphiboles. Phys Chem Miner 29:562–570
Rivalenti G, Garuti G, Rossi A (1975) The origin of the Ivrea-Verbano basic formation (Western Italian Alps), whole rock chemistry. Boll Soc Geol Ital 94:1149–1186
Rivalenti G, Rossi A, Siena F, Sinigoi S (1984) The layered series of the Ivrea-Verbano igneous complex, Western Alps, Italy. Tscher Miner Petrol 33:77–99
Robinson P (1982) Phase relations of metamorphic amphiboles: natural occurrence and theory. Rev Miner 9B:1–3
Rothkirch A, Gatta GD, Meyer M, Merkel S, Merlini M, Liermann H-P (2013) Single-crystal diffraction at the Extreme conditions beamline P02.2: procedure for collecting and analyzing high-pressure single-crystal data. J Synchrotron Rad 20:711–720
Schmidt MW, Poli S (1998) Experimentally based water budgets for dehydrating slabs and consequences for arc magma generation. Earth Planet Sci Lett 163:361–379
Shen G, Liermann H-P, Sinogeikin S, Yang W, Hong X, Yoo C-S, Cynn H (2007) Distinct thermal behavior of GeO2 glass in tetrahedral, intermediate, and octahedral forms. Proc Natl Acad Sci 104:14576–14579
Siena F, Coltorti M (1989) The petrogenesis of a hydrated mafic ultramafic complex and the role of amphibole fractionation at Finero(Italian Western Alps). Neues Jahrb Miner Monatsh 6:255–274
Stern RJ (2002) Subduction zones. Rev Geophys 40:1012. doi:10.1029/2001RG000108
Sueno S, Cameron M, Papike JJ, Prewitt CT (1973) High temperature crystal chemistry of tremolite. Am Mineral 58:649–664
Syracuse EM, van Keren PE, Abers GA (2010) The global range of subduction zone thermal models. Phys Earth Planet Int 183:73–90
Thompson P, Cox DE, Hastings JB (1987) Rietveld refinement of Debye–Scherrer synchrotron X-ray data of Al2O3. J Appl Cryst 20:79–83
Thompson EC, Campbell AJ, Liu ZX (2016) In-situ infrared spectroscopic studies of hydroxyl in amphiboles at high pressure. Am Mineral 101:706–712
Tribaudino M, Bruno M, Iezzi G, Della Ventura G, Margiolaki I (2008) The thermal behavior of richterite. Am Mineral 93:1659–1665
Vannucci R, Piccardo GB, Rivalenti G, Zanetti A, Rampone E, Ottolini L, Oberti R, Mazzucchelli M, Bottazzi P (1995) Origin of LrEE-depleted amphiboles in the subcontinental mantle. Geochim Cosmochim Acta 59:1763–1771
Wallace ME, Green DH (1991) The effect of bulk rock composition on the stability of amphibole in the upper mantle: implications for solidus positions and mantle metasomatism. Miner Pet 44:1–19
Welch MD, Knight KS (1999) A neutron powder diffraction study of cation ordering in high-temperature synthetic amphiboles. Eur J Miner 11:321–331
Welch MD, Camara F, Della Ventura G, Iezzi G (2007) Non-ambient in situ studies of amphiboles. Rev Miner Geochem 67:223–260
Welch MD, Reece JJ, Redfern SAT (2008) Rapid intracrystalline exchange of divalent cations in amphiboles: a high-temperature neutron diffraction study of synthetic K-richterite AKB(NaCa) C(Mg2.5Ni2.5)Si8O22(OH)2. Miner Mag 72:877–886
Welch MD, Gatta GD, Rotiroti N (2011a) The high-pressure behavior of orthorhombic amphiboles. Am Mineral 96:623–630
Welch MD, Cámara F, Oberti R (2011b) Thermoelasticity and high-T behaviour of anthophyllite. Phys Chem Miner 38:321–334
Yang H, Hazen RM, Prewitt CT, Finger LW, Lu R, Hemley RJ (1998) High-pressure single-crystal X-ray diffraction and infrared spectroscopic studies of the C2/m-P21/m phase transition in cummingtonite. Am Mineral 83:288–299
Zanazzi PF, Nestola F, Pasqual D (2010) Compressibility of protoamphibole: a high-pressure single-crystal diffraction study of protomangano-ferro-anthophyllite. Am Mineral 95:1758–1764
Zema M, Welch MD, Oberti R (2012) High-T behaviour of gedrite: thermoelasticity, cation ordering and dehydrogenation. Contrib Miner Pet 163:923–937
Zhang L, Ahsbahs H, Kutoglu A, Hafner SS (1992) Compressibility of grunerite. Am Mineral 77:480–483
Acknowledgements
Mario Tribaudino and Wilson Crichton are gratefully thanked for the useful and fruitful comments and suggestions. The Editor, Milan Rieder is acknowledged for handling the manuscript. PETRA-III synchrotron facility (Hamburg, Germany) is acknowledged for provision of beamtime at P02.2 beamline. ELETTRA (Trieste, Italy) synchrotron facility is acknowledged for beamtime at MCX beamline. J. Plaisier is thanked for the support during the experiment at ELETTRA. The authors acknowledge the University of Milano, the Doctoral School of Earth Science of the University of Milano, and the DCO (Deep Carbon Observatory) for supporting the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comboni, D., Lotti, P., Gatta, G.D. et al. Pargasite at high pressure and temperature. Phys Chem Minerals 45, 259–278 (2018). https://doi.org/10.1007/s00269-017-0915-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-017-0915-0