Abstract
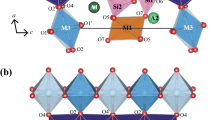
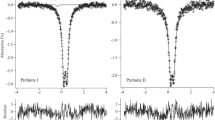
A vauxite mineral sample from Huanuni, Bolivia, was studied by XRD, TGA and Mössbauer spectroscopy. The XRD revealed the sample as having the typical triclinic structure of vauxite. The chemical formula was determined as (Fe0.88Mn0.01)Al1.99(PO4)2(OH)1.75(H2O)5.31, implying some Fe2+, OH− and H2O deficiencies. The TGA curve showed ca. 27% loss of weight over a temperature range from 80 to 400 °C, supposedly due to the loss of water and hydroxyl groups. For the first time, Mössbauer spectra for vauxite were collected over a wide temperature range between 9 and 310 K. No magnetic ordering was detected. The spectra could be successfully and consistently analyzed by a superposition of four doublet subspectra. On the basis of the relation between the center shift and the mean Fe-ligand distance on the one hand and the center shift values for the various doublets on the other hand, one doublet was assigned to Fe(2). For the other doublets, it is proposed that, as a result of the H2O deficiency in the structure of the present vauxite sample, vacancies are present in the second coordination spheres of some Fe(1) and that these vacancies affect the quadrupole splitting of the corresponding Fe(1) cations, thus causing three Fe(1) doublet components in the Mössbauer spectra. The temperature variations of center shift and quadrupole splitting of the various doublet contributions are presented and discussed.






Similar content being viewed by others
References
Ballhausen CJ (1962) Introduction to ligand field theory. McGraw-Hill Book Company Inc, New-York
Baur WH (1969) The crystal structure of paravauxite, Fe2+Al2(PO4)2(OH)2(OH2)6.2H2O. Neues Jahrbuch fur Mineralogie, Monatshefte:430-433
Baur WH, Rama Rao B (1967) The crystal structure of metavauxite. Die Naturwissenschaften 54:561
Baur WH, Rama Rao B (1968) The crystal structure and the chemical composition of vauxite. Am Miner 53:1025–1028
De Grave E, Van Alboom A (1991) Evaluation of ferrous and ferric Mössbauer fractions. Phys Chem Miner 18:337–342
Eeckhout SG, De Grave E (2003a) Evaluation of ferrous and ferric Mössbauer fractions: Part II. Phys Chem Miner 30:142–146
Eeckhout SG, De Grave E (2003b) Fe-57 Mössbauer-effect studies of Ca-rich, Fe-bearing clinopyroxenes: Part I. Paramagnetic spectra of magnesian hedenbergite. Am Miner 88:1129–1137
Ericsson T, Wäppling R (1976) Texture effects in 3/2 → 1/2 Mössbauer spectra. J Phys Colloq C6(37):719–723
Freeman AJ, Watson RE (1963) Antishielding of magnetic and electric hyperfine interactions in open shell ions. Phys Rev 131:2566–2573
Greneche JM, Varret F (1982) On the texture problem in Mossbauer spectroscopy. J Phys C Solid State Phys 15:5333–5344
Ingalls R (1964) Electric-field gradient tensor in ferrous compounds. Phys Rev 133:787–795
Lauer S, Marathe VR, Trautwein A (1979) Sternheimer shielding using various approximations. Phys Rev A 19:1852–1861
Pound RV, Rebka GA Jr (1960) Variation with temperature of the energy of recoil free gamma rays from solids. Phys Rev Lett 4:274–277
Sternheimer RM (1972) Quadrupole shielding and antishielding factors for several atomic ground states. Phys Rev A 6:1702–1709
Van Alboom A, De Resende VG, De Grave E, Gomez JM (2009) Hyperfine interactions in szomolnokite (FeSO4·H2O). J Mol Struct 924–926:448–456
Van Alboom A, De Grave E, Wohlfahrt-Mehrens M (2011) Temperature dependence of the Fe2+ Mössbauer parameters in triphylite (LiFePO4). Am Miner 96(2–3):408–416
Van Alboom A, De Resende VG, da Costa GM, De Grave E (2015) Mössbauer spectroscopic study of natural eosphorite, (Mn, Fe)AlPO4(OH)2H2O. Am Miner 100(2–3):580–587
Ventruti G, Schingaro E, Monno A, Lacalamita M, Della Ventura G, Bellatreccia F, Cuocci C, Rossi M, Capitelli F (2016) Structure refinement and vibrational spectroscopy of vauxite from the type locality, Llallagua (Bolivia). Can Mineral 54:163–176
Zhao M, Du M (1983) Two-center transitions in the anti-ferromagnetic salt FeCO3. Phys Rev B 28(11):6481–6484
Acknowledgements
This work was funded by the Fund for Scientific Research—Flanders, Belgium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Alboom, A., da Costa, G.M. & De Grave, E. Deficiency of water molecules in the crystallographic structure of vauxite. Phys Chem Minerals 45, 249–257 (2018). https://doi.org/10.1007/s00269-017-0913-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00269-017-0913-2