Abstract
Objective
Postoperative intra-abdominal infection is one of the most serious complications after pancreatic resection. In this article, we investigated the relationship between serum lactate level and postoperative infection, to suggest a new predictor of potential infection risk after pancreatectomy.
Methods
A retrospective analysis of 156 patients who underwent pancreatic surgery and admitted in the intensive care unit for recovery after surgery between August 2017 and August 2019 was performed.
Results
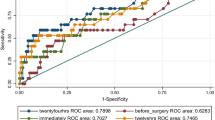
The basic characteristics, preoperative information, pathological diagnoses, surgical methods, and intraoperative situations of patients in the postoperative intra-abdominal infection group (n = 52) and non-infection group (n = 104) showed no significant differences. With the same postoperative treatments and results of fluid balance, blood pressure maintenance, and laboratory tests, postoperative serum lactate level increased much higher in the infection group than non-infection group (P < 0.001), while the base excess level declined much lower (P = 0.002). Patients in the infection group needed more time to elute lactate (P < 0.001), and stayed longer in the intensive care unit after surgery (P = 0.007). The overall postoperative complications were certainly more in the infection group (P < 0.001), resulting in a longer hospitalization time (P < 0.001).
Conclusions
When patients recovered smoothly from anesthesia with a stable hemodynamics situation and normal results of laboratory tests, abnormally high serum lactate level could be a predictor of postoperative intra-abdominal infection after pancreatic resection.


Similar content being viewed by others
References
Harrison LE, Brennan MF (1998) Portal vein resection for pancreatic adenocarcinoma. Surg Oncol Clin N Am 7(1):165–181
Johnson CD et al (1993) Resection for adenocarcinoma of the body and tail of the pancreas. Br J Surg 80(9):1177–1179
Li YT et al (2019) Effect of Blumgart anastomosis in reducing the incidence rate of pancreatic fistula after pancreatoduodenectomy. World J Gastroenterol 25(20):2514–2523
Li Y et al (2017) Comparison of long-term benefits of organ-preserving pancreatectomy techniques for benign or low-grade malignant tumors at the pancreatic head. Medicine 96(51):e9420 (Baltimore)
Strasberg SM, Drebin JA, Linehan D (2003) Radical antegrade modular pancreatosplenectomy. Surgery 133(5):521–527
Whipple AO (1941) The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann Surg 114(4):612–615
Kawai M, Yamaue H (2010) Analysis of clinical trials evaluating complications after pancreaticoduodenectomy: a new era of pancreatic surgery. Surg Today 40(11):1011–1017
Oguro S et al (2017) Three hundred and sixty-eight consecutive pancreaticoduodenectomies with zero mortality. J Hepatobiliary Pancreat Sci 24(4):226–234
Zhang Z, Xu X (2014) Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review and meta-analysis*. Crit Care Med 42(9):2118–2125
Zhang Z, Xu X, Chen K (2014) Lactate clearance as a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review study protocol. BMJ Open 4(5):e004752
Haas SA et al (2016) Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensiv Care Med 42(2):202–210
de Vries HM, Dekker SE, Boer C (2014) Lactate clearance as a predictor of mortality. J Trauma Acute Care Surg 77(1):183
Freitas AD, Franzon O (2015) Lactate as predictor of mortality in polytrauma. Arq Bras Cir Dig 28(3):163–166
Odom SR et al (2013) Lactate clearance as a predictor of mortality in trauma patients. J Trauma Acute Care Surg 74(4):999–1004
Gu WJ, Zhang Z, Bakker J (2015) Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensiv Care Med 41(10):1862–1863
Hayashida K et al (2017) Early lactate clearance is associated with improved outcomes in patients with postcardiac arrest syndrome: a prospective, multicenter observational study (SOS-KANTO 2012 Study). Crit Care Med 45(6):e559–e566
Jones AE et al (2010) Lactate clearance versus central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA 303(8):739–746
Hajjar LA et al (2013) High lactate levels are predictors of major complications after cardiac surgery. J Thorac Cardiovasc Surg 146(2):455–460
Lindsay AJ et al (2013) Lactate clearance time and concentration linked to morbidity and death in cardiac surgical patients. Ann Thorac Surg 95(2):486–492
Zhou BC et al (2018) Blood lactate or lactate clearance: Which is robust to predict the neurological outcomes after cardiac arrest? A systematic review and meta-analysis. Biomed Res Int 2018:8014213
Weil MH, Afifi AA (1970) Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 41(6):989–1001
Bassi C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years after. Surgery 161(3):584–591
Sugiura T et al (2012) Risk factor of surgical site infection after pancreaticoduodenectomy. World J Surg 36(12):2888–2894. https://doi.org/10.1007/s00268-012-1742-6
Callery MP et al (2013) A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 216(1):1–14
Bassi C et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138(1):8–13
Bassi C et al (2010) Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg 252(2):207–214
Fujii T et al (2014) Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 18(6):1108–1115
Wente MN et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS). Surgery 142(5):761–768
Wente MN et al (2007) Postpancreatectomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition. Surgery 142(1):20–25
Vin Y et al (2008) Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg 207(4):490–498
Lee J, Little TD (2017) A practical guide to propensity score analysis for applied clinical research. Behav Res Ther 98:76–90
Reiffel JA (2018) Propensity-score matching: optimal, adequate, or incomplete? J Atr Fibrillation 11(4):2130
Grendar J et al (2017) Validation of fistula risk score calculator in diverse North American HPB practices. HPB (Oxford) 19(6):508–514
Okabayashi T et al (2005) Postoperative pancreatic fistula following surgery for gastric and pancreatic neoplasm; is distal pancreaticosplenectomy truly safe? Hepatogastroenterology 52(61):233–236
Shi N et al (2016) Splenic preservation versus splenectomy during distal pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol 23(2):365–374
Liu Z et al (2019) Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand J Trauma Resusc Emerg Med 27(1):51
Ryoo SM et al (2018) Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med 46(6):e489–e495
Shankar-Hari M et al (2016) Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8):775–787
Singer M et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8):801–810
Polk HJ, Lopez-Mayor JF (1969) Postoperative wound infection: a prospective study of determinant factors and prevention. Surgery 66(1):97–103
(2012) Antimicrobial prophylaxis for surgery. Treat Guidel Med Lett 10(122):73–8; quiz 79–80
Bratzler DW et al (2013) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 14(1):73–156 (Larchmt)
Kasatpibal N et al (2017) Failure to redose antibiotic prophylaxis in long surgery increases risk of surgical site infection. Surg Infect 18(4):474–484 (Larchmt)
Mitchell NJ, Evans DS, Pollock D (1980) Pre-operation single-dose cefuroxime antimicrobial prophylaxis with and without metronidazole in elective gastrointestinal surgery. J Antimicrob Chemother 6(3):393–399
Okamura K et al (2017) Randomized controlled trial of perioperative antimicrobial therapy based on the results of preoperative bile cultures in patients undergoing biliary reconstruction. J Hepatobiliary Pancreat Sci 24(7):382–393
Funding
This work was supported by the project of Capital’s Funds for Health Improvement and Research (2020-1-4011), and the project of application and promotion of capital special clinical research from Beijing Municipal Science & Technology Commission (Z171100001017017018).
Author information
Authors and Affiliations
Contributions
YL and LC are the co-first authors of this article, who took in charge of data collection, analysis, article writing and language editing; CX, CD, HZ, and SW helped to collect data; MD is the correspondence author of this article, who guided the whole work, while YZ, YL, JG, QL, and TZ helped to conduct the research.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yatong Li and Lixin Chen are co-first authors.
Rights and permissions
About this article
Cite this article
Li, Y., Chen, L., Xing, C. et al. Changes in Serum Lactate Level Predict Postoperative Intra-Abdominal Infection After Pancreatic Resection. World J Surg 45, 1877–1886 (2021). https://doi.org/10.1007/s00268-021-05987-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-05987-8