Abstract
Background
Management of the post-traumatic open abdomen (OA) using negative pressure wound therapy (NPWT) alone is associated with low rates of primary fascial closure. The abdominal reapproximation anchor (ABRA) system exerts dynamic medial fascial traction and may work synergistically with NPWT to facilitate primary fascial closure.
Methods
Patients with an OA following trauma laparotomy between 2009 and 2018 were identified from a prospectively maintained institutional database. Patients treated with ABRA in conjunction with NPWT (ABRA) versus NPWT alone (NPWT) were compared in terms of primary fascial closure rate, number of surgeries to closure, tracheostomy duration, length of stay and incidence of entero-atmospheric fistula. Multivariable linear regression was performed to identify predictors of tracheostomy duration.
Results
We identified 48 patients [ABRA, 12 and NPWT, 36]. The ABRA group was significantly younger (25 vs. 37 years, p = 0.027) and included a lower proportion of males (58% vs. 89%, p = 0.032). Groups were similar with respect to the incidence of hollow viscus injury, injury severity score and abdominal abbreviated injury score. Compared to the NPWT group, the ABRA group had a significantly higher rate of primary fascial closure (100% vs. 28%, p < 0.001), fewer surgeries to abdominal closure (2 vs. 2.5, p = 0.023) and shorter duration of tracheostomy (15.5 vs. 36 days, p = 0.008). There were no differences in length of stay or incidence of entero-atmospheric fistula. On multivariable linear regression, ABRA placement was an independent predictor of shorter tracheostomy duration, after adjusting for covariates (β = − 0.294, p = 0.036).
Conclusion
For the post-traumatic OA, ABRA coupled with NPWT achieves a higher rate of primary fascial closure compared to NPWT alone, while requiring fewer surgeries and a shorter duration of tracheostomy.
Similar content being viewed by others
Introduction
Damage control laparotomy (DCL) significantly improves the survival of trauma patients with exsanguinating abdominal injuries [1, 2]. DCL with temporary abdominal closure (TAC) allows for expedited transfer to an intensive care unit (ICU) and prompt correction of coagulopathy, hypothermia and acidosis [3]. Following the control of life-threatening injuries, patients experience significant morbidity related to their open abdomen (OA) [4]. Traditional management with planned ventral hernia and delayed abdominal wall reconstruction may be associated with aesthetically unsightly and functionally incapacitating complications, such as entero-atmospheric fistula, chronic back pain, impaired bowel function and reduced mobility [5, 6].
Achieving early primary fascial closure is key to mitigate the morbidity associated with prolonged OA [7]. Multiple methods have been developed to facilitate primary fascial approximation. Traditional techniques, such as the Bogota bag and Wittmann Patch, have been largely replaced by negative pressure wound therapy (NPWT) [8,9,10]. NPWT systems, such as ABThera (KCI Inc., San Antonio, TX, USA), generate continuous centripetal negative pressure, which promotes fascial approximation and evacuation of the inflammatory exudate [11]. Despite these advantages, we and others have shown that approximately 30% of patients managed with NPWT alone fail to achieve primary fascial closure [5, 12, 13].
Dynamic fascial traction systems may work synergistically with NPWT to improve primary fascial closure rates. The abdominal reapproximation anchor system (ABRA; Canica Design Inc, Almonte, ON, Canada) is a novel device which exerts dynamic appositional traction through transfascial elastomers. Despite encouraging reported fascial closure rates, the literature remains limited to small, descriptive case series, with a heterogeneity of indications [14,15,16,17]. Herein, we report our institutional outcomes for ABRA coupled with NPWT, compared to NPWT alone, following trauma DCL. To our knowledge, this comparative study is the first of its kind in a trauma population to date.
Methods
Patient selection
We identified all patients who underwent damage control laparotomy with an open abdomen at a single level I trauma centre between 2009 and 2018. Demographic and peri-operative data, including injury severity score (ISS) [18], abdominal abbreviated injury score (AAIS), mechanism of trauma and presence of hollow viscus injury, were collected from a prospectively maintained institutional trauma database. The primary outcome was primary fascial closure rate. Secondary outcomes included time to abdominal closure, number of surgeries to abdominal closure, length of hospital stay, duration of tracheostomy, rate of delayed abdominal wall reconstruction (AWR) and incidence of entero-atmospheric fistula (EAF). This study was approved by the institutional review board of the McGill University Health Centre.
When OA serves as a bridge to second-look laparotomy, with definitive repair performed within 24–48 h, fascial closure does not typically pose a technical challenge. However, certain patients develop fascial retraction and/or intra-abdominal oedema, which preclude abdominal closure at the time of definitive repair surgery. To capture this specific patient subset, we defined our inclusion criteria as follows: patients who did not undergo abdominal closure at the time of definitive repair surgery [8]. We considered definitive repair surgery to be the first operation during which haemostasis was achieved (without surgical packing), and intestinal continuity was restored (or stoma was created). We excluded patients whose abdomen was closed at the time of definitive repair surgery and who died prior to abdominal closure.
For example, suppose a patient initially undergoes DCL with liver packing and intestinal resection (left in discontinuity). The patient undergoes a second surgery to remove the haemostatic packing, followed by a third surgery to perform the intestinal anastomosis. If abdominal closure was performed at the time of the third (definitive repair) surgery, this patient would be excluded. However, if the abdomen was left open at the third surgery, following completion of the intestinal anastomosis, the patient would be included in the study. Study patients were divided into two groups: ABRA in conjunction with NPWT (hereafter referred to as “ABRA group”) versus NPWT alone (hereafter referred to as “NPWT group”).
Surgical technique
The traditional practice at our institution was to use NPWT alone, either the Barker vacuum pack [19] or the VAC system (KCI Inc., San Antonio, TX, USA), for temporary abdominal closure. The technique for VAC installation has been previously described [20]. Dressing changes were performed every 3–5 days in the ICU or the operating theatre. The ABRA system became available at our institution in 2016. Thereafter, the decision to use ABRA with NPWT versus NPWT alone was at the discretion of the attending surgeon.
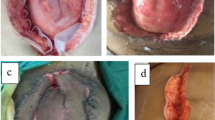
For ABRA installation, a perforated silicone sheet is placed over the viscera to prevent adhesions to the abdominal wall, to protect the viscera from the elastomers and to allow for the abdominal wall to glide medially with increased tension of the elastomers. A series of midline-crossing elastomers are inserted perpendicular to the fascia, through the full thickness of the abdominal wall, approximately 5 cm from the medial fascial margin. These elastomers are placed approximately 3 cm apart and secured into button anchors, which self-adhere onto the abdominal skin. The elastomers are positioned into slits on a tubular silicone retainer, which maintains their alignment (Fig. 1a). The elastomers are tightened until the calibration markers are stretched to 1.5–2 times their untensioned length, with the highest tension around the middle of the wound. The ABThera polyurethane sponge and adhesive dressing are placed over the elastomers in the wound and connected to the negative pressure pump (Fig. 1b). The elastomers are tightened daily at the bedside, stretching the markings to twice their original length to gradually appose the wound edges. When deemed feasible, primary fascial closure is performed in the operating theatre.
Installation of the ABRA dynamic closure system. (a) Transfascial elastomers are secured into button anchors, and alignment is maintained using a silicone retainer. (b) ABThera polyurethrane sponge and adhesive dressing are placed over the elastomers, and connected to a negative pressure pump. The patient consented to the publication of these images
Statistical analysis
Continuous variables, expressed as median [range], were compared using the Mann–Whitney U test. Categorical variables were compared using the Pearson’s Chi-square test. To generate the matched cohort, ABRA and NPWT cases were matched 1:1 based on predetermined variables that were deemed biologically relevant: age (closest match, within 20 years), hollow viscus injury (exact match) and AAIS (closest match, within 5 points). Multivariable linear regression was used to evaluate the association between TAC technique and tracheostomy duration, with adjustment for age, hollow viscus injury, ISS and AAIS. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software, version 22 (SPSS Inc., Chicago, IL, USA).
Results
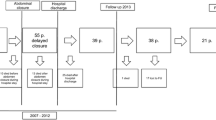
From 2009 to 2018, 147 trauma patients underwent DCL with an OA. Ninety-nine patients were excluded because abdominal closure was performed at the time of definitive repair surgery (n = 68), or because of death prior to abdominal closure (n = 31). Of 48 patients included in the study, 12 underwent ABRA placement in conjunction with NPWT, while 36 were managed with NPWT alone. The median time to ABRA placement was 6 days. Following the introduction of ABRA in 2016, all patients included in the study were managed with ABRA in conjunction with NPWT. All patients included in the NPWT alone group were treated prior to the institutional availability of ABRA. Of the patients managed with NPWT alone, four were treated with VAC therapy, whereas the remainder was managed with the Barker vacuum pack.
Demographic and peri-operative outcomes are summarized in Table 1. The ABRA group was significantly younger (25 vs. 37 years, p = 0.027), with a lower proportion of males (58% vs. 89%, p = 0.032). ISS, AAIS and incidence of hollow viscus injury were comparable between the two groups. Of the patients who sustained a hollow viscus injury, one patient (14%) in the ABRA group and four patients (25%) in the NPWT group developed a leak that required surgical drainage or reanastomosis.
The ABRA group had a significantly higher rate of primary fascial closure (100% vs. 28%, p < 0.001) and required fewer surgeries to achieve abdominal closure (2 vs. 2.5, p = 0.023). Of patients who achieved primary fascial closure, those in the ABRA group were significantly less likely to require component separation (0% vs. 50%, p = 0.010). Twenty-six patients in the NPWT group underwent non-fascial closure: skin only (n = 17); split-thickness skin graft (n = 6); and Vicryl mesh (n = 3). Although both groups had similar tracheostomy rates, the duration of tracheostomy was significantly shorter in the ABRA patients (15.5 vs. 36 days, p = 0.008). The median time to tracheostomy insertion was 10 days in the ABRA group vs. 12 days in the NPWT group. There were no differences in duration of OA, duration of mechanical ventilation, ICU length of stay, rate of delayed AWR or incidence of EAF. The ABRA group had a trend towards shorter length of hospital stay compared to NPWT alone (46 vs. 74 days, p = 0.055), but this was not statistically significant. No patients in the ABRA group developed complications related to skin necrosis. The 90-day mortality rates were 0% in the ABRA group, compared to 5.6% in the NPWT group.
Outcomes for the matched cohort [ABRA, 12; NPWT, 12] are summarized in Table 2. The ABRA group redemonstrated a higher rate of primary fascial closure (100% vs. 12%, p < 0.001), fewer surgeries to abdominal closure (2 vs. 4.5, p = 0.008) and shorter duration of tracheostomy (15.5 vs. 35 days, p = 0.017), compared to NPWT alone. Ventilator duration was also significantly shorter in the ABRA group (20 vs. 34.5 days, p = 0.045).
On multivariable linear regression analysis, ABRA placement (β = –0.294, p = 0.036), age (β = 0.333, p = 0.021) and presence of hollow viscus injury (β = 0.329, p = 0.020) were independent predictors of tracheostomy duration, after adjusting for covariates (Table 3).
Discussion
Damage control surgery has improved the survival of patients with life-threatening traumatic injuries [1, 21]. However, the resulting open abdomen presents distinct management challenges. Prolonged OA leads to fascial retraction and adhesions to the abdominal wall, which may ultimately preclude fascial closure. Failure to achieve primary fascial closure is associated with increased morbidity, decreased quality of life and higher costs related to AWR [4, 22]. Optimizing TAC techniques to achieve early primary fascial closure is an integral tenet of OA management. Evidence-based consensus on the most effective technique for OA management following trauma DCL is lacking. Our institution had mainly used NPWT systems alone, but approximately 30% of all-comer post-traumatic OA cases failed to achieve primary fascial closure. With the advent of the ABRA system, we sought to compare outcomes of patients treated with ABRA in conjunction with NPWT versus NPWT alone.
We observed a significantly higher rate of primary fascial closure in the ABRA group (100% vs. 28%), which is consistent with data reported in other series [14, 16, 17]. The low rate of primary fascial closure in the NPWT alone group is attributable to our criteria to only include patients whose OA could not be closed at the time of definitive repair surgery. This effectively excluded patients for whom OA was simply a bridge to definitive repair surgery and who would have had a higher likelihood of primary fascial closure, irrespective of TAC technique. Interestingly, among patients who achieved primary fascial closure, the ABRA group required a significantly lower rate of component separation (0% vs. 50%, p = 0.010). Given the considerable morbidity associated with component separation, obviating the need for additional myofascial mobilization is an important benefit [23]. Furthermore, the ABRA group required fewer surgeries to achieve abdominal closure (2 vs. 2.5 unmatched, 2 vs. 4.5 matched). Of note, our median time to ABRA placement (6 days) was relatively short compared to other studies [14, 16, 17]. Early application of a dynamic traction device, prior to the onset of irreversible fascial retraction, may increase the likelihood of achieving primary fascial closure.
The ABRA group required fewer delayed abdominal wall reconstructions (8% vs. 36%). While awaiting AWR, large abdominal wall defects may result in paradoxical respiratory motion and impair pulmonary mechanics. In contrast, tightening of the ABRA elastomers may increase intra-abdominal pressure to an extent that hinders respiratory mechanics. Thus, we used tracheostomy duration as a surrogate to evaluate the effect of ABRA versus NPWT on overall respiratory dynamics. We observed a significantly shorter duration of tracheostomy in ABRA patients (15.5 vs. 36 days). Further, ABRA placement was an independent predictor of shorter tracheostomy duration on multivariable linear regression. These results suggest that ABRA may not have a detrimental effect on intra-abdominal pressure, and may improve respiratory mechanics and expedite return of normal physiology by facilitating early primary fascial closure.
Some authors have suggested that NPWT itself may predispose to the development of entero-atmospheric fistulas [24]. In our study, none of the ABRA patients developed an EAF. In contrast, EAF occurred in 14% of patients treated with NPWT alone, which is consistent with published data on both NPWT and non-negative pressure techniques [25,26,27,28]. Indeed, there are multiple risk factors for EAF, including prolonged open abdomen, mechanical irritation of wound dressings, poor nutritional status and inflammatory bowel disease [29]. Thus, although it is challenging to quantify the risk associated with NPWT itself, achieving early definitive fascial closure remains a key element to minimize the risk of developing EAF.
In an era of value-based care, the adoption of novel proprietary technologies must be justified based on improved patient outcomes [30, 31]. Our study suggests that the higher costs of the ABRA system may be partially offset by savings incurred from fewer surgeries to closure, decreased need for delayed AWR, shorter tracheostomy duration and fewer entero-atmospheric fistulae. A formal cost-effectiveness analysis, accounting for in-hospital outcomes and post-discharge quality of life metrics, will be important to justify the higher upfront costs of ABRA.
This study is limited by its retrospective design. In the absence of an institutional protocol, TAC management was at the discretion of the attending surgeon. To mitigate potential selection bias, we generated an ABRA:NPWT subgroup, which was closely matched on predetermined variables that were deemed to be biologically relevant. On matched analysis, the ABRA group redemonstrated a significantly higher primary fascial closure rate, fewer number of surgeries to closure and shorter tracheostomy duration, consistent with the unmatched comparison. Importantly, following the introduction of ABRA in 2016, all patients who met our study inclusion criteria were managed with ABRA in conjunction with NPWT. All patients in the NPWT alone group were treated prior to the institutional availability of ABRA, thus effectively serving as a “historical” control. This likely reflects surgeons’ tendency to reserve ABRA for patients with bona fide open abdomen management issues. Admittedly, there has been an evolution towards restrictive fluid administration and early haemostatic resuscitation, which may affect fascial closure rates. However, our current institutional massive transfusion protocol was implemented in 2008, prior to the start of the study. There have been no significant changes to this resuscitation protocol over the study period. To further illustrate this point, we divided patients from the pre-ABRA era (2009–2016) into two time periods: 2009–2012 versus 2013–2016. There were no differences in fascia closure rates (29.4% vs. 26.3%, p = 1.00) and time to abdominal closure (11 vs. 10 days, p = 0.75) between the two groups. Together, this suggests that the different fascial closure rates between our two groups likely reflect the TAC technique (ABRA vs. NPWT), rather than changes in resuscitation strategies over the 9-year study period.
Despite its relatively small sample size, this study represents the largest comparative series in the trauma literature to date. In addition, our cohort was homogeneous, as all patients underwent DCL for abdominal trauma. Other groups have typically combined a variety of indications (trauma, intra-abdominal sepsis, necrotizing pancreatitis, ruptured abdominal aortic aneurysm) [14, 17]. This renders data interpretation challenging, as certain patient populations may respond preferentially to dynamic fascial traction [27]. To this end, the International Register of Open Abdomen (IROA) registry found that patients with peritonitis treated with NPWT had better survival compared to non-negative pressure techniques [27]. However, in this patient population, NPWT was associated with the lowest fascial closure rate, at only 54%. In trauma patients, there was no survival difference between NPWT and non-negative pressure techniques. However, the fascial closure rate with NPWT was the highest at 78%. These findings highlight the importance of patient selection and suggest a distinct underlying pathophysiology in trauma patients that may make them more likely to benefit from a combined negative pressure and dynamic fascial traction approach. Prospective randomized trials and large-scale international registries will help further refine patient selection and treatment algorithms.
Conclusion
In trauma patients with an OA, dynamic fascial traction coupled with NPWT achieves a higher rate of primary fascial closure compared to NPWT alone. Evidence-based patient selection and management algorithms are necessary to optimize its efficacy.
References
Rotondo MF et al (1993) ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 35:375–382
Stone HH, Strom PR, Mullins RJ (1983) Management of the major coagulopathy with onset during laparotomy. Ann Surg 197:532–535
Morris JA, Eddy VA, Blinman TA, Rutherford EJ, Sharp KW (1993) The staged celiotomy for trauma. Issues in unpacking and reconstruction. Ann Surg 217:576–584
Miller RS, Morris JA, Diaz JJ, Herring MB, May AK (2005) Complications after 344 damage-control open celiotomies. J Trauma 59:1365–1371
Wang Y et al (2018) Incidence and factors associated with development of heterotopic ossification after damage control laparotomy. Injury 49:51–55
Nieuwenhuizen J, Halm JA, Jeekel J, Lange JF (2007) Natural course of incisional hernia and indications for repair. Scand J Surg 96:293–296
Hatch QM et al (2011) Impact of closure at the first take back: complication burden and potential overutilization of damage control laparotomy. J Trauma 71:1503–1511
Weinberg JA et al (2008) Closing the open abdomen: improved success with Wittmann Patch staged abdominal closure. J Trauma 65:345–348
Tieu BH et al (2008) The use of the Wittmann Patch facilitates a high rate of fascial closure in severely injured trauma patients and critically ill emergency surgery patients. J Trauma 65:865–870
Mattox KL (1997) Introduction, background, and future projections of damage control surgery. Surg Clin N Am 77:753–759
Ribeiro Junior MAF et al (2016) Open abdomen in gastrointestinal surgery: which technique is the best for temporary closure during damage control? World J Gastrointest Surg 8:590–597
Cheatham ML et al (2013) Prospective study examining clinical outcomes associated with a negative pressure wound therapy system and Barker's vacuum packing technique. World J Surg 37:2018–2030
Cirocchi R et al (2016) What is the effectiveness of the negative pressure wound therapy (NPWT) in patients treated with open abdomen technique? A systematic review and meta-analysis. J Trauma Acute Care Surg 81:575–584
Mukhi AN, Minor S (2014) Management of the open abdomen using combination therapy with ABRA and ABThera systems. Can J Surg 57:314–319
Verdam FJ et al (2011) Delayed primary closure of the septic open abdomen with a dynamic closure system. World J Surg 35:2348–2355
Haddock C, Konkin DE, Blair NP (2013) Management of the open abdomen with the abdominal reapproximation anchor dynamic fascial closure system. Am J Surg 205:528–533
Reimer MW et al (2008) Management of open abdominal wounds with a dynamic fascial closure system. Can J Surg 51:209–214
Baker SP, O'Neill B, Haddon W, Long WB (1974) The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 14:187–196
Brock WB, Barker DE, Burns RP (1995) Temporary closure of open abdominal wounds: the vacuum pack. Am Surg 61:30–35
Olona C et al (2014) Comparative study of open abdomen treatment: ABThera™ vs. abdominal dressing™. Hernia 19:323–328
Lee JC, Peitzman AB (2006) Damage-control laparotomy. Curr Opin Crit Care 12:346–350
Kritayakirana K et al (2010) Outcomes and complications of open abdomen technique for managing non-trauma patients. J Emerg Trauma Shock 3:118–122
Desai NK et al (2016) Open repair of large abdominal wall hernias with and without components separation; an analysis from the ACS-NSQIP database. Ann Med Surg 7:14–19
Richter S, Dold S, Doberauer JP, Mai P, Schuld J (2013) Negative pressure wound therapy for the treatment of the open abdomen and incidence of enteral fistulas: a retrospective bicentre analysis. Gastroenterol Res Pract 2013:6
Rasilainen SK, Mentula PJ, Leppäniemi AK (2012) Vacuum and mesh-mediated fascial traction for primary closure of the open abdomen in critically ill surgical patients. Br J Surg 99:1725–1732
Bradley MJ et al (2013) Independent predictors of enteric fistula and abdominal sepsis after damage control laparotomy: results from the prospective AAST open abdomen registry. JAMA Surg 148:947–954
Coccolini F et al (2017) IROA: international register of open abdomen, preliminary results. World J Emerg Surg 12:10–10
Coccolini F et al (2019) Open abdomen and entero-atmospheric fistulae: an interim analysis from the international register of open abdomen (IROA). Injury 50:160–166
Lynch AC et al (2004) Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg 240:825–831
Porter ME (2010) What is value in health care? N Engl J Med 363:2477–2481
Isik A et al (2016) Effectiveness of manual knotting at laparoscopic appendectomy. Gazi Med J 27:19–20
Funding
None.
Author information
Authors and Affiliations
Contributions
The study was designed by YW, AA, TP, AB, PF, KK, TR, JG and DD; data were collected by YW, AA and TP; data were analysed by YW, AA, JG and DD; data were interpreted by YW, AA, JG and DD; manuscript was written by YW, AA, JG and DD; and critical revision was carried out by YW, AA, TP, AB, PF, KK, TR, JG and DD.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Alnumay, A., Paradis, T. et al. Management of Open Abdomen After Trauma Laparotomy: A Comparative Analysis of Dynamic Fascial Traction and Negative Pressure Wound Therapy Systems. World J Surg 43, 3044–3050 (2019). https://doi.org/10.1007/s00268-019-05166-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05166-w