Abstract
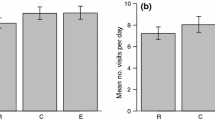
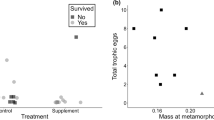
One way parents shape the fitness prospects of their offspring is by providing a nursery. When parents divide a brood across several nurseries, they must assess not only the costs and benefits of multiple nurseries, but also the optimal proportion of the brood to deposit in each. Here, we explored the factors shaping these decisions in the strawberry poison frog (Oophaga pumilio), a species that typically, but not always, deposits its offspring singly in small phytotelmata. By monitoring occupancy and tadpole success in an artificial phytotelm array used by a free-living population, we tested for preferences for nursery size and height, asked whether multi-tadpole deposition was non-random with respect to these factors, and assessed the fitness consequences of these decisions. Parents were equally likely to use all types of artificial phytotelmata, but multi-tadpole depositions occurred almost exclusively in large nurseries. The probability that a tadpole would complete metamorphosis was unrelated to the physical characteristics of the rearing site, and individual tadpole survival was equivalent regardless of whether the nursery held one, two, or three tadpoles. Multi-tadpole nurseries were more likely, therefore, to have one occupant surviving to independence (metamorphosis). Although tadpoles are usually deposited alone, O. pumilio mothers may benefit from the insurance function played by additional tadpoles in a nursery.
Significance statement
While most animals that care for their young provide a single nursery for their entire brood, a few separate and rear each brood member in an individual nursery. In such cases, parents must decide not only which nurseries to use, but also how many young they should deposit into each. Here, we explore the factors shaping these decisions in the strawberry poison frog (Oophaga pumilio), a species that typically, but not always, rears its young individually in small water-filled leaf axils. While O. pumiliodo not appear to choose nurseries based on size, parents were more likely to deposit multiple tadpoles into larger nurseries. Additionally, because the number of tadpoles in a nursery did not influence likelihood of metamorphic success for each individual, nurseries that contained multiple tadpoles were more likely to produce at least one offspring that completed metamorphosis. These results highlight the fitness effects of plasticity in reproductive strategies, and the possibility of divergent parent and offspring fitness optima.



Similar content being viewed by others
References
van Alphen JJM, Visser ME (1990) Superparasitism as an adaptive strategy for insect parasitoids. Annu Rev Entomol 35:59–79
Barclay MR (1988) Variation in the costs, benefits, and frequency of nest reuse by barn swallows (Hirundo rustica). Auk 105:53–60
Brown JL, Morales V, Summers K (2008) Divergence in parental care, habitat selection, and larval life history between two species of Peruvian poison frogs: an experimental analysis. J Evol Biol 21:1534–1543
Brown JL, Morales V, Summers K (2010) A key ecological trait drove the evolution of biparental care and monogamy in an amphibian. Am Nat 175:436–446
Brust DG (1990) Maternal brood care by Dedrobates pumilio: a frog that feeds its young. PhD dissertation. Cornell University, Ithaca
Buxton VL, Sperry JH (2017) Reproductive decisions in anurans: a review of how predation and competition affects the deposition of eggs and tadpoles. BioScience 67:26–38
Cayuela H, Lengagne T, Kaufmann B, Joly P, Léna JP (2016) Larval competition risk shapes male-male competition and mating behaviour in an anuran. Behav Ecol:1726–1733
Donnelly MA (1989) Effects of resource supplementation on space-use patterns in Dendrobates pumilio. Oecologia 81:212–218
Dugas MB (2018) Simple observations with complex implications: what we have learned and can learn about parental care from a frog that feeds its young. Zool Anz 273:192–202
Dugas MB, Wamelink CL, Killius AM, Richards-Zawacki CL (2016a) Parental care is beneficial for offspring, costly for mothers, and limited by family size in an egg-feeding frog. Behav Ecol 27:476–483
Dugas MB, Stynoski J, Strickler SA (2016b) Larval aggression is independent of food limitation in nurseries of a poison frog. Behav Ecol Sociobiol 70:1389–1395
Dugas MB, Moore MP, Martin RA, Richards-Zawacki CL, Sprehn CG (2016c) The pay-offs of maternal care increase as offspring develop, favouring extended provisioning in an egg-feeding frog. J Evol Biol 29:1977–1985
Dugas MB, Strickler SA, Stynoski JL (2017) Tadpole begging reveals high quality. J Evol Biol 30:1024–1033
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Fincke O (1999) Organization of predator assemblages in Neotropical tree holes: effects of abiotic factors and priority. Ecol Entomol 24:13–23
Gomez-Mestre I, Pyron RA, Wiens JJ (2012) Phylogenetic analyses reveal unexpected patterns in the evolution of reproductive modes in frogs. Evolution 66:3687–3700
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Holdridge LR (1971) Forest environments in tropical life zones. Pergamon, Oxford
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev 72:283–327
Khazan ES, Bright EG, Beyer JE (2015) Land management impacts on tree hole invertebrate communities in a Neotropical rainforest. J Insect Conserv 19:681–690
Khazan ES, Arias M, Fernández LM (2016) Large mammal community composition under a disturbance gradient in Northeast Costa Rica. Rev Biol Trop 64:1553–1564
Lehtinen RM (ed) (2004) Ecology and evolution of phytotelm-breeding anurans. Misc Publ Mus Zool Univ Mich 193:1–73
Lewis LT, Grant P, Garćia Quesada M, Ryall C, LaDuke TC (2010) A botanical survey of Caño Palma Biological Station (Estación Biológica Caño Palma), Tortuguero, Costa Rica. Brenesia 73(74):73–84
Magnusson WE, Hero JB (1991) Predation and the evolution of complex oviposition behaviour in Amazon rainforest frogs. Oecologia 86:310–318
Maple MM (2002) Maternal effects on offspring fitness in Dendrobates pumilio, the strawberry poison frog. PhD dissertation. University of Kentucky, Lexington
McKeon CS, Summers K (2013) Predator driven reproductive behavior in a tropical frog. Evol Ecol 27:725–737
Meuche I, Linsenmair E, Prӧhl E (2011) Female territoriality in the strawberry poison frog (Oophaga pumilio). Copeia 2011:351–356
Mock DW (1985) Siblicidal brood reduction: they prey-size hypothesis. Am Nat 125:327–343
Mock DW, Forbes LS (1995) The evolution of parental optimism. Trends Ecol Evol 10:130–134
Mock DW, Parker GA (1997) The evolution of sibling rivalry. Oxford University Press, Oxford
Morey S, Reznick D (2000) A comparative analysis of plasticity in larval development in three species of spadefoot toads. Ecology 81:1736–1749
Poelman EH, Dicke M (2007) Offering offspring as food to cannibals: oviposition strategies of Amazonian poison frogs (Dendrobates ventrimaculatus). Evol Ecol 21:215–227
Poelman EH, van Wijngaarden RPA, Raaijmakers CE (2013) Amazon poison frogs (Ranitomeya amazonica) use different phytotelm characteristics to determine their suitability for egg and tadpole depositions. Evol Ecol 27:661–674
Pröhl H, Berke O (2001) Spatial distributions of male and female strawberry poison frogs and their relation to female reproductive success. Oecologia 129:534–542
Pröhl H, Hödl W (1999) Parental investment, potential reproductive rates, and mating system in the strawberry dart-poison frog, Dendrobates pumilio. Behav Ecol Sociobiol 46:215–220
Refsnider JM, Janzen FJ (2010) Putting eggs in one basket: ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu Rev Ecol Evol S 41:39–57
Resetaris WJ, Wilbur H (1989) Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology 70:220–228
Ringler E, Mangione R, Ringler M (2015) Where have all the tadpoles gone? Individual genetic tracking of amphibian larvae until adulthood. Mol Ecol Resour 15:737–746
Ringler E, Szipl G, Harrigan RJ, Bartl-Binder P, Mangione R, Ringler M (2018) Hierarchical decision-making balances current and future reproductive success. Mol Ecol 27:2289–2301
Rojas B (2014) Strange parental decisions: fathers of the dyeing poison frog deposit their tadpoles in pools occupied by large cannibals. Behav Ecol Sociobiol 68:551–559
Royle NJ, Smiseth PT, Köliker M (eds) (2012) The evolution of parental care. Oxford University Press, Oxford
Ryan MJ, Barry DS (2011) Competitive interactions in phytotelmata—breeding pools of two poison-dart frogs (Anura: Dendrobatidae) in Costa Rica. J Herpetol 45:438–443
Schulte LM (2010) Preference and competition for breeding plants in coexisting Ranitomeya species (Dendrobatidae): does height play a role? Salamandra 46:180–184
Schulte LM, Yeager JD, Schulte R, Veith M, Werner P, Beck LA, Lӧtters S (2011) The smell of success: choice of larval rearing sites by means of chemical cues in a Peruvian poison frog. Anim Behav 81:1147–1154
Schwagmeyer PL, Mock DW (2008) Parental provisioning and offspring fitness: size matters. Anim Behav 75:291–298
Stearns SC (1992) The evolution of life-histories. Oxford University Press, Oxford
Stynoski JL, Shelton G, Stynoski P (2014) Maternally derived chemical defences are an effective deterrent against some predators of poison frog tadpoles (Oophaga pumilio). Biol Lett 10:20140187
Summers K, McKeon CS (2004) The evolutionary ecology of phytotelmata use in neotropical poison frogs. Misc Publ Mus Zool Univ Mich 193:55–73
Touchon JC, Warkentin KM (2008) Reproductive mode plasticity: aquatic and terrestrial oviposition in a treefrog. P Natl Acad Sci USA 105:7495–7499
Wells KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago
Acknowledgements
We thank Charlotte Foale and Manuel Arias for logistical support. Gijs Bouwemeester, David Boeren, Emily Simmonds, Jess Sutton, and Nick Humphreys assisted with field work. Alexander T. Baugh and two anonymous reviewers provided comments that greatly improved the quality of this manuscript.
Funding
The Canadian Organization for Tropical Education and Rainforest Conservation provided partial funding for this work, and support was also provided by HAS Hogeschool’s internship program for the biological sciences (to TV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. The Ministerio de Ambiente, Energía y Telecomunicaciones of Costa Rica approved all methods and issued the appropriate permit (ACTO-PIN-014-2015).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. T. Baugh
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 40 kb)
Rights and permissions
About this article
Cite this article
Khazan, E.S., Verstraten, T., Moore, M.P. et al. Nursery crowding does not influence offspring, but might influence parental, fitness in a phytotelm-breeding frog. Behav Ecol Sociobiol 73, 33 (2019). https://doi.org/10.1007/s00265-019-2642-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2642-7