Abstract
Eusociality is characterised by a reproductive division of labour, where some individuals forgo direct reproduction to instead help raise kin. Socially polymorphic sweat bees are ideal models for addressing the mechanisms underlying the transition from solitary living to eusociality, because different individuals in the same species can express either eusocial or solitary behaviour. A key question is whether alternative social phenotypes represent environmentally induced plasticity or predominantly genetic differentiation between populations. In this paper, we focus on the sweat bee Lasioglossum calceatum, in which northern or high-altitude populations are solitary, whereas more southern or low-altitude populations are typically eusocial. To test whether social phenotype responds to local environmental cues, we transplanted adult females from a solitary, northern population, to a southern site where native bees are typically eusocial. Nearly all native nests were eusocial, with foundresses producing small first brood (B1) females that became workers. In contrast, nine out of ten nests initiated by transplanted bees were solitary, producing female offspring that were the same size as the foundress and entered directly into hibernation. Only one of these ten nests became eusocial. Social phenotype was unlikely to be related to temperature experienced by nest foundresses when provisioning B1 offspring, or by B1 emergence time, both previously implicated in social plasticity seen in two other socially polymorphic sweat bees. Our results suggest that social polymorphism in L. calceatum predominantly reflects genetic differentiation between populations, and that plasticity is in the process of being lost by bees in northern populations.
Significance statement
Phenotypic plasticity is thought to play a key role in the early stages of the transition from solitary to eusocial behaviour, but may then be lost if environmental conditions become less variable. Socially polymorphic sweat bees exhibit either solitary or eusocial behaviour in different geographic populations, depending on the length of the nesting season. We tested for plasticity in the socially polymorphic sweat bee Lasioglossum calceatum by transplanting nest foundresses from a northern, non-eusocial population to a southern, eusocial population. Plasticity would be detected if transplanted bees exhibited eusocial behaviour. We found that while native bees were eusocial, 90% of transplanted bees and their offspring did not exhibit traits associated with eusociality. Environmental variables such as time of offspring emergence or temperatures experienced by foundresses during provisioning could not explain these differences. Our results suggest that the ability of transplanted bees to express eusociality is being lost, and that social polymorphism predominantly reflects genetic differences between populations.
Similar content being viewed by others
Introduction
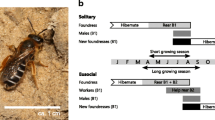
There is increasing interest in the environmental and genetic mechanisms underlying the transition from solitary living to eusociality (e.g. Yanega 1997; Field et al. 2010, 2012; Kapheim et al. 2012, 2015a; Kocher et al. 2013; Rehan and Toth 2015), and investigating these mechanisms requires taxa that straddle this transition (Field et al. 2010; Rehan and Toth 2015). Socially polymorphic sweat bees (Hymenoptera: Halictidae) are ideal models for this purpose, because different populations of the same species exhibit either eusocial or solitary behaviour (Soucy and Danforth 2002; Chapuisat 2010). In spring, mated females (foundresses) emerge from hibernation and excavate individual nest burrows. Each foundress then mass provisions a first brood (B1) of offspring in separate, sealed brood cells. In solitary populations, B1 offspring emerge to mate and females enter hibernation, becoming the following year’s new foundresses. In eusocial populations, however, at least some B1 females become workers that instead help to rear a second brood (B2) of reproductive offspring (Schwarz et al. 2007). Season length is thought to be a key proximate constraint on social phenotype because eusociality can be expressed only where the season is long enough to rear two consecutive broods (Soucy and Danforth 2002; Hirata and Higashi 2008; Field et al. 2010; Davison and Field, in preperation)
Within sweat bees, there have been at least two origins of eusociality and many subsequent losses, including to social polymorphism (Danforth 2002; Brady et al. 2006; Gibbs et al. 2012). It is thought that such reversals could be driven by selection acting on only a small number of regulatory switches (West-Eberhard 2003), and that in transitional populations initial plasticity in social phenotype might be lost once environmental conditions become predictable (Field et al. 2010; Cini et al. 2015; Smith et al. 2015). Therefore, a key question is to what extent alternative eusocial and solitary phenotypes result from environmentally mediated plasticity or represent distinct, genetically fixed alternatives (Wcislo 1997).
Field transplants are critical to addressing this question and yet are rarely performed (Yanega 1997; Field et al. 2012). Reciprocal transplants of the socially polymorphic sweat bee Halictus rubicundus Christ between social and solitary populations in the UK revealed that social phenotype is plastic with respect to the environment (Field et al. 2010, 2012). Evidence suggests that B1 females become workers only when emerging sufficiently early in the season (Field et al. 2010; see also Hirata and Higashi 2008), and that nest foundresses may be able to adjust the size of B1 offspring depending on anticipated social phenotype (Field et al. 2012, but see Field et al. 2010). Conversely, significant mitochondrial differentiation exists between eusocial and solitary populations of North American H. rubicundus, suggesting that social phenotype might have a fixed genetic component (Soucy and Danforth 2002). A laboratory common garden experiment also suggested that social phenotype may have a fixed genetic component in Lasioglossum albipes Fabricius (Plateaux-Quénu et al. 2000). However, fixed genetic differences between social phenotypes have never been demonstrated experimentally in a natural field setting, which is critical to fully account for unmeasured environmental variables (Yanega 1997; Field et al. 2012).
Lasioglossum calceatum Scopoli is a common and widespread socially polymorphic sweat bee of the Palearctic, closely related to L. albipes (Sakagami and Munakata 1972; Pesenko et al. 2000; Danforth et al. 2003; Davison and Field 2016). In this paper, we test for plasticity in social phenotype by transplanting foundresses from a northern, solitary UK population to a southern UK population where native bees are typically eusocial (see Davison and Field 2016 for site details). We also genotype offspring, which confirms previous work suggesting that L. calceatum expresses eusociality in the south of the UK (Davison and Field 2016).
We focus on three aspects of B1 female social phenotype: emergence time, pollen collection and body size. B1 offspring might become workers only if they emerge sufficiently early in the season (Hirata and Higashi 2008; Field et al. 2010), workers typically begin provisioning the natal nest within 1 or 2 days of emergence (PJD, personal observation), and in common with most other eusocial sweat bees, B1 workers tend to be smaller than their mothers (Packer and Knerer 1985; Davison and Field 2016). Critically, since offspring are mass provisioned, B1 body size may largely reflect investment decisions by foundresses at the time of provisioning (Plateaux-Quénu 1983; Richards and Packer 1994). If social phenotype is plastic, foundresses transplanted to the south from northern solitary populations might respond by producing small B1 offspring that in turn remain at their nest as workers, or perhaps initiate their own nests instead of entering hibernation (Field et al. 2010, 2012). Instead, we find that most transplanted foundresses and their offspring show no evidence of plasticity, indicating that inter-population differences in social phenotype predominantly reflect genetic differentiation.
Methods
Transplants
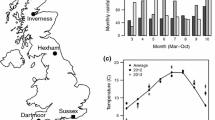
Foundresses were transplanted from Inverness, in the far north of the UK, approximately 800 km south to a nesting aggregation at the University of Sussex campus (Sussex) where annual temperatures are higher and the season is longer (Fig. 1; Table 1). Bees at Inverness nest solitarily but bees at Sussex typically express eusociality (Davison and Field 2016). Because nesting aggregations are hard to find (Richards et al. 2015), we were unable to perform a control transplant from another eusocial site in the present study. However, controls implemented in previous studies show that transplantation per se is unlikely to influence behaviour (Field et al. 2010, 2012; Davison and Field, in preparation). Bees were transplanted from Inverness on 15–16 August 2014 (autumn transplant) and 16 May 2015 (spring transplant). Autumn-transplanted bees (n = 70) were freshly emerged B1 females caught returning to their nests from feeding/mating flights. Spring-transplanted bees (n = 202) were nest foundresses recently emerged from hibernation, and were caught returning from feeding or provisioning flights. Most spring transplants were not carrying pollen and were therefore unlikely to have already begun provisioning their own offspring.
Bees were caught with an insect net and marked on both the clypeus and thorax with a single spot of enamel paint (Revell® and Humbrol™ enamel model paints), denoting the time of transplant (autumn or spring). Thus, transplanted bees could be readily distinguished from native Sussex bees. Prior to release, bees were maintained inside individual plastic tubes either in a cold box containing ice packs or a fridge at 4 °C. Autumn-transplanted bees were released directly into 14 L plastic buckets containing artificial nest holes (15–20 cm), which were embedded into the ground near to the nesting aggregation at Sussex. Each bucket was covered with netting to encourage bees to enter the holes. Netting was removed the following morning and bees allowed to fly freely inside an insect-proof cage containing flowers before entering hibernation inside the buckets, which were left embedded in the ground throughout the winter. Buckets were removed and re-embedded within the Sussex nest aggregation before the start of spring 2015. Spring-transplanted bees were released over three evenings (17–19 May 2015) directly into artificial nest holes created among native and autumn-transplanted nests within the Sussex aggregation (not in the buckets). Of the two autumn-transplanted bees that successfully founded nests (see “Results”), one nested within a bucket and one in the ground surrounding the buckets. Similarly, some spring-transplanted foundresses also founded nests in buckets, while others utilised the artificial nest burrows or dug new nest burrows in the surrounding soil.
Foundress demography and body size
We recorded the timing of three key events for native and transplanted bees: (1) date of nest initiation (the first day on which a foundress provisioned), (2) date of first B1 female emergence and (3) the time taken to produce the first B1 female offspring (time between (1) and (2)).
Foundresses initiating nests in spring were caught with an insect net after being observed provisioning, and individually marked with unique colour combinations of two enamel paint spots (Revell® and Humbrol™) applied to the thorax with a pin. Wing length was measured to the nearest 0.1 mm with digital callipers, as the distance between the outer edge of the tegula and the end of the forewing. Nest entrances were marked using individually numbered nails. During the foundress provisioning phase, the nesting aggregation was divided into two sections, which were observed continuously by the same person on alternate days when the weather was suitable for foraging (n = 29 observation days). It was not possible to record data blind because our study involved focal animals in the field.
The timing of nest initiation and offspring emergence date can vary between years, and therefore as a comparison we utilise demographic data from two previous years in which native L. calceatum were studied at Sussex: 2012 and 2013. Data in these years were collected using the same methods as in the present study (see Davison and Field 2016 for details).
Determining social phenotype and offspring size
Nests were considered eusocial only if B1 females were observed provisioning. Workers were identified as unmarked bees provisioning a nest in which the foundress had been marked: workers begin provisioning within 1 or 2 days of emergence, whereas B1 females that directly enter hibernation are typically observed entering the nest for several days after emergence but never with pollen (Davison and Field 2016). B1 females were caught on emergence from their nest only after being observed provisioning. They were then measured and marked with a single paint spot on the clypeus and thorax, using a colour unique within their nest. Directly hibernating offspring were measured when excavated from beneath their nests at the end of the season (see below). With the help of a second observer, nests in both sections were continually observed during the worker-provisioning phase (n = 26 observation days).
Nest excavations
Nests were excavated from 6 to 15 August 2015, near to the end of the season but prior to the emergence of B2 offspring (Fig. 2). All brood and adult bees (B1 females and foundresses) were removed and stored in ethanol before genotyping. In nests of L. calceatum, cells forming each brood are arranged in a single cluster surrounded by a cavity (Sakagami and Michener 1962), and it was therefore possible to be certain that all brood in a given nest had been collected. Excavations were continued well below the level of brood cell clusters to detect hibernating B1 offspring.
The timing and duration of key events for spring-transplanted Inverness (light grey) and native Sussex (dark grey) nest foundresses and their offspring. Solid bars show the periods during which activity was observed, and represent all bees in that cohort. Not all bees within each cohort, represented by a bar, began or finished individual stages on the same day. Bars therefore represent the first and last days on which different individual bees within a cohort were observed. Gaps between bars shows periods of bee inactivity
DNA extraction and microsatellite analysis
DNA was extracted from whole bees using the ammonium acetate precipitation method (Nicholls et al. 2000). We amplified 12 microsatellite loci, originally developed for L. malachurum, in two multiplexes (Parsons et al. 2017; Table S1). Multiplexes were amplified in a 2 μL Qiagen Multiplex reaction using the following PCR profile: 95 °C for 15 min, followed by 44 × (94 °C for 30 s, 57 °C for 90 s and 72 °C for 60 s), then 60° for 30 min. PCR products were genotyped using an ABI 3730 48-well capillary DNA Analyser using LIZ size standard (Applied Biosystems Inc.), and alleles were scored using Genemapper® v3.7 software. Two microsatellites were monomorphic and were discarded. The remaining ten loci had 6–19 alleles per locus (\( \overline{x} \) = 9.5 alleles per locus) across both populations (see Table S2 for breakdown by population). We tested for linkage disequilibrium (LD) and departure from Hardy-Weinberg equilibrium (HW) within and among the Sussex and Inverness populations using Genepop 4.2 (Raymond and Rousset 1995; Rousset 2008). For these tests, we selected one female from each nest at Sussex to avoid pseudoreplication. To correct p values in multiple tests, we applied the q value to LD p values using QVALUE (Storey 2002). The q value is a measure of significance in terms of false discovery rate unlike the conventional Bonferroni correction, which attempts to measure significance in terms of false positives only (Storey 2002), and therefore provides a more powerful method for correcting multiple tests (Verhoeven et al. 2005). As a measure of genetic diversity, we recorded the total number of alleles at a given locus, and the observed and expected heterozygosity.
Brood relatedness
We used the software Relatedness 5.0.8 (Queller and Goodnight 1989) to estimate the life-for-life coefficient of relatedness (r) among B2 females and between foundresses and B2 females within each nest. Allele frequencies were estimated and calculations performed when weighting nests equally. Standard errors and 95% confidence intervals were obtained by jackknifing over nests (Queller and Goodnight 1989). We separated female B2 brood into groups of full sisters using the computer program Kinship 1.3.1 (Goodnight and Queller 1999). We asked whether females were more likely to be full sisters (r = 0.75) than aunt-niece (r = 0.375), with 100,000 replicates to estimate significance values: any B2 female brood laid by a B1 female will be the niece of a B2 female brood laid by the foundress. Where the principle egg-layer’s genotype was available, or could be reconstructed, we assigned male production: males were allocated to the principle egg-layer if they shared one of her two alleles at each locus, and to a secondary female if one or more alleles were not shared.
Confirming offspring population of origin
Lasioglossum offspring typically hibernate beneath their natal nest (Sakagami and Fukuda 1972), and B1 offspring of transplanted foundresses were indeed frequently found hibernating beneath their natal nests. In nests where we were unable to match the genotypes of B1 offspring with the foundress, however, we used STRUCTURE (version 2.3.4; Pritchard et al. 2000) to confirm that these hibernating adults were not offspring of native foundresses that might have entered the nest. STRUCTURE divides genotypes into genetic clusters according to HW and LD. Using this method, we could also test whether two of three bees (the third was not genotyped) that initiated new nests during the worker-provisioning phase originated from native or transplanted nests. We assumed admixture and uncorrelated allele frequencies, and specified the number of possible genetic clusters as K = 1–3. We ran three replicates for each K, and specified a burn-in period of 100,000 steps. A single individual from each nest was included, together with additional adults caught at Inverness in spring 2015 but not released at Sussex (n = 21) and additional bees from the Sussex native population (n = 18). We implemented the Evanno method (Evanno et al. 2005) within the program Structure Harvester (Earl and Vonholdt 2012) to determine the best fitting value of K. We further characterised genetic differentiation between our Inverness and Sussex L. calceatum populations by calculating FST using the default settings in Genepop 4.2 (Raymond and Rousset 1995; Rousset 2008).
Data analysis
To determine whether transplanted foundresses and their offspring exhibited plasticity, we examined two characteristics associated with social phenotype: worker behaviour and B1 offspring size.
First, we tested whether the observed pattern of behaviour exhibited by offspring of native and transplanted foundresses indicated (i) environmentally mediated plasticity or (ii) fixed genetic differences between the two populations. Under plasticity, the timing of B1 emergence might be a key factor mediating the decision of B1 offspring to become workers (Field et al. 2010). A significant effect of ‘source’ (Sussex or Inverness) on social phenotype would indicate fixed genetic differences between populations, whereas a significant effect of ‘emergence date’ would be indicative of plasticity. Spring-transplanted foundresses typically provisioned to produce their B1 offspring later than native foundresses (Fig. 2; Fig. 5a), and therefore could have experienced different environmental conditions that may have influenced offspring social phenotype. To control for one such factor, we included temperature during a foundresses’ provisioning period in the model. This was calculated as the average of mean daily temperature for each day between a foundress’s first and last observed provisioning events, yielding ‘foundress provisioning temperature’. We analysed the effect of ‘source’, ‘emergence date’ (designated as the date on which the first B1 female emerged at each nest) and ‘foundress provisioning temperature’ on a nest’s ‘phenotype’ (presence or absence of workers) using a generalised linear model (GLM) with binomial errors. Given that later-provisioned offspring also emerged later (Fig. 5a), we checked for collinearity among explanatory variables (Dormann et al. 2013) by examining variance inflation factor (VIF) scores using the function ‘vif’ in the R-Package ‘car’ (Fox and Weisberg 2011). We employed a conservative threshold of VIF ≥ 2.5 to identify collinearity (Allison 2012). For all variables, VIF scores were low (< 1.3), indicating no significant collinearity.
Second, because eusociality in L. calceatum is associated with B1 offspring (workers) that are significantly smaller than their mothers (caste-size dimorphism; Davison and Field 2016), we tested for caste-size dimorphism and examined whether ‘source’ affected the size of B1 female offspring produced by native and transplanted foundresses. As there were multiple offspring per nest, we used a generalised linear mixed model (GLMM) to test for effects of ‘caste’ and ‘source’ on ‘female wing length’, with ‘nest’ included as a random factor. We initially included a caste/source interaction to test whether foundresses from different sources produced offspring of different sizes relative to themselves.
We also tested for differences between native and transplanted bees in the time taken to produce the first B1 offspring. We used a GLM with normal errors to test for effects of ‘first foundress provision date’ and ‘source’ on ‘development time’. Development time was considerably left skewed, and so we implemented the function powerTransform in the R-package ‘car’ (Fox and Weisberg 2011) to transform the data. Offspring of transplanted and native foundresses were different in size, and therefore we included ‘size’ to control for this difference. As a measure of size, we used the mean wing length of marked workers in eusocial nests, and of B1 females excavated at the end of the season from solitary nests.
We used a GLM with negative binomial errors to examine the relationship between the number of workers in a nest and ‘productivity’ (the number of immature B2 offspring produced), with ‘number of workers’ as the single explanatory variable.
We used Chi-squared tests with Yates’ correction to compare the frequency of nest failure between nests initiated by native versus transplanted foundresses, and to compare the frequency of successful spring nest initiation between autumn and spring-transplanted Inverness bees. Foundresses were considered to have successfully initiated nests once they had started provisioning, and nest failure was indicated by the absence of detected B1 offspring.
For all models, we report significance values when removing terms from the minimal adequate model, after stepwise reduction from the maximal model (Crawley 2013). All analyses were conducted in the R environment (R Development Core Team 2013). Results are presented ± 1 standard error.
All data generated or analysed during this study are included in this published article and its supplementary information files.
Results
Nesting success of transplanted bees
Spring and autumn-transplanted foundresses were equally likely to initiate nests at Sussex (Pearson’s chi-squared test: X2 = 0.862, p = 0.353, n = 4/70 autumn-transplanted (5.7%), n = 21/202 spring-transplanted (10.4%)). Of these, approximately half successfully produced B1 offspring (n = 2/4 autumn-transplanted, n = 10/21 spring-transplanted). The rate of failure to produce at least one detected B1 offspring did not differ between nests initiated by native and transplanted foundresses (Pearson’s chi-squared test: X2 = 0.019, p = 0.891, native = 56.6%, n = 53 nests; transplanted = 52.4%, n = 25).
Social phenotype
Social phenotype was successfully recorded at 39 nests (n = 29 native, n = 10 spring-transplanted). Nearly all native nests (n = 28/29) were social, with B1 female offspring that began provisioning as workers (\( \overline{x} \) = 3.1 ± 0.33 B1 workers per nest). In contrast, nine out of ten nests initiated by spring-transplanted Inverness foundresses did not become social (Fig. 4a). The two successful nests initiated by autumn-transplanted foundresses also did not become social: one produced two B1 males and the other a single B1 female that brought one recorded pollen load to the nest before disappearing. Although social phenotype was somewhat ill defined at these nests, neither scenario was observed among the 29 native nests.
Foundresses were alive at the time of B1 female emergence in six of the nine solitary nests initiated by spring-transplanted foundresses, and these foundresses were regularly observed leaving the nest on nectar-collecting trips alongside B1 females. The single transplanted foundress whose nest became social produced three B1 females, all of which began provisioning in the presence of the foundress. Provisioning behaviour by B1 females was clear-cut at this nest because each B1 female was observed provisioning on at least ten occasions over three or more separate days (Yanega 1989). In contrast with the solitary nests, and in common with native eusocial nests, the foundress did not leave her nest after B1 offspring emergence. Among native nests, the number of B2 offspring produced increased linearly with the number of workers in a nest (GLM: X21,15 = 4.944, p = 0.026; Fig. 3). The single eusocial nest initiated by a spring-transplanted foundress produced just two B2 offspring, despite having three B1 provisioners. Three native nests containing three workers produced three, five, and ten B2 offspring, respectively (Fig. 3).
Relationship between the number of provisioning workers recorded a nest and the number of B2 male and female offspring produced. Filled circles represent native nests, and the filled triangle the single social transplanted nest. Points are jittered to reveal multiple overlapping data points. The dashed line shows least-squares regression for native nests
Bee size
Native foundresses produced B1 females significantly smaller than themselves. In contrast, transplanted foundresses produced offspring the same size as themselves, and which were larger than native B1 females (GLMM: caste/source interaction X21 = 20.302, p < 0.001; Fig. 4b). Two of the three B1 offspring produced in the single eusocial nest initiated by a transplanted foundress were the same size as native workers, while the third was closer in size to the mean for transplanted foundress’ B1 offspring. A B1 female offspring excavated from beneath the single native solitary nest was similar in size to other native B1 females that became workers. All of these offspring are included in the analysis illustrated in Fig. 4b.
a Proportion of native nests at Sussex (Sx) and nests initiated by spring-transplanted foundresses from Inverness (Iv) that expressed social or solitary behaviour. b Mean wing lengths (mm) of native (Sussex) and transplanted (Inverness) foundresses and their B1 female offspring (± 1SE). Foundresses: n = 18 from Sussex, n = 5 from Inverness. B1 females: n = 51 from Sussex, n = 13 from Inverness. Significant caste/source interaction X21 = 20.302, p < 0.001
Foundress provisioning, offspring emergence date and development time
Native Sussex foundresses were first observed provisioning on 20 April 2015, with an average first provisioning date of 24 April ± 1.4 days (n = 51 native foundresses). However, because the season started later in Inverness than at Sussex in 2015, and because spring transplants could not be carried out until foundresses emerged from hibernation in spring, the first spring-transplanted foundresses did not begin provisioning until 20 May (Fig. 2; Fig. 5a). Nevertheless, two native Sussex foundresses did begin provisioning after spring-transplanted foundresses (Fig. 5a) and had eusocial nests. Moreover, four spring-transplanted foundresses that did not produce workers still began provisioning before the latest-provisioning eusocial foundresses in 2012 and 2013 (Fig. 5b). The two autumn-transplanted foundresses that established nests began provisioning on 21 April and 9 May, respectively.
Social phenotype and the relationship between the date a foundress was first observed to provision in spring and the emergence date of her first female offspring. Panels show a data from the present study only and b data from the present study together with data gathered in the same way by Davison and Field (2016) for comparison, representing native bees studied in 2012 and 2013. Symbols denote social phenotype and source: open symbols show social nests where at least some offspring became workers, and filled symbols solitary nests where no female B1 offspring became workers. Triangles denote bees transplanted from Inverness, and circles and squares native bees from Sussex. See keys for details. Points are jittered to reveal multiple overlapping observations. Day zero is 14 April and day 70 is 23 June, standardised across years
Time taken to produce the first female offspring did not differ between nests initiated by native and transplanted foundresses, and decreased linearly as the date when a foundress first started provisioning progressed (GLM: F1,30 = 292.58, p < 0.001). Nevertheless, because their nests were generally initiated later in spring, the first female offspring of transplanted foundresses emerged later than those from most native nests (Fig. 5a; Wilcoxon signed rank test: W = 27.5, p < 0.001, n = 10 transplanted, n = 31 native nests), although at an earlier date than almost all native offspring in two previous years (Fig. 5b). However, nests of transplanted bees may not have been solitary simply because B1 offspring emerged later in the season, or because transplanted foundresses provisioned later in the spring: four nests of native foundresses had been initiated later, or had B1 females that emerged later, yet still became eusocial (Fig. 5a). Indeed, even after controlling for the effects of temperature during foundress provisioning, and for offspring emergence date, nests initiated by native foundresses were still significantly more likely to become eusocial than nests initiated by transplanted foundresses (GLM: foundress provisioning temperature X21,34 = 0.739, p = 0.390; B1 emergence date X21,34 = 1.613, p = 0.204; bee source X21,34 = 5.565, p = 0.021). Additionally, B1 female offspring of transplanted foundresses in the present study emerged relatively early compared with native eusocial B1 offspring from two previous years in the same nest aggregation at Sussex (Fig. 5b; Davison and Field 2016).
Brood genotyping
Prior to q value correction, three locus pairs were weakly significant for LD. After q value correction, however, there was no significant LD within or between the Sussex and Inverness populations. One locus deviated from HW at both Sussex and Inverness (LMA53, see Table S2). Genetic diversity was variable among loci, with a mean expected heterozygosity of 0.66 at Sussex and 0.54 at Inverness (Table S2).
We successfully genotyped B2 offspring from 22 nests (n = 15 native, n = 7 transplanted). Native nests contained a mean of 5.7 genotyped B2 offspring per nest (\( \overline{x} \) = 3.7 ± 0.67 females and \( \overline{x} \) = 1.9 ± 0.41 males). Five nests also contained a live foundress and five contained live workers at the time of excavation. Genetic data confirm our behavioural observations that L. calceatum exhibits eusociality at Sussex: average relatedness among B2 female brood within nests was 0.74 ± 0.03 (mean ± SE; 95% CI [0.67; 0.81]) (see Table 2 for a breakdown by nest). In four of five nests containing a live foundress at excavation, the foundress monopolised most or all B2 reproduction (Fig. 6; Table 2). Of native nests containing multiple B2 female brood (n = 13/15), approximately half (n = 6/13) contained a single B2 female which was not sister to the remaining female brood (Fig. 6). Six of 12 nests in which it was possible to assign males contained males not laid by the principal egg-layer (Fig. 6; Table S3). We found no evidence of multiple mating by native foundresses, suggesting that Sussex L. calceatum are monandrous. However, we found evidence of at least one alien bee reproducing within a single nest (see nest 58 in Table 2).
Partitioning of B2 offspring produced in social nests initiated by native foundresses at Sussex. Each nest is represented by a bar, and offspring from different mothers are represented by blocks within each bar. Asterisks indicate nests in which the foundress was alive at the time of excavation (see Table 2 and Table S3 for details)
We successfully genotyped the foundress, a marked B1 female and two female B2 offspring from the single eusocial nest initiated by a spring-transplanted Inverness foundress. The foundress was the mother of the B1 female. However, the two female B2 offspring were not sisters, and our data suggest two alternative possibilities for parentage: (i) the foundress mated multiply and laid both, or (ii) a B1 female laid one. In either case, bees in this nest exhibited eusocial behaviour not previously recorded at Inverness (Davison and Field 2016). We also genotyped female B1 offspring from six solitary nests of transplanted foundresses (\( \overline{x} \) = 1.8 per nest). In the nests where we genotyped the foundress, she matched as mother to most or all of the adult females excavated from her nest. The population of origin for B1 females in the remaining four nests was determined by the STRUCTURE analysis (see below).
The STRUCTURE analysis strongly supported the existence of the two known populations (K = 2, Sussex and Inverness), and assigned all bees of known origin to the correct cluster (Fig. S1). The pairwise FST value for our Inverness and Sussex was 0.286, indicating considerable genetic differentiation between our populations (Soro et al. 2010). All B1 offspring excavated from beneath the nests of transplanted foundresses were assigned to the cluster containing bees from Inverness. The two genotyped bees that initiated new nests in the summer were assigned to the Sussex population. Independent summer nest founding has not previously been reported (Davison and Field 2016), and therefore represents the discovery of a new behaviour by L. calceatum at Sussex.
Discussion
Few studies have utilised field transplants to address the mechanisms underlying socially polymorphic behaviour (Field et al. 2010, 2012, see also Cronin 2001; Baglione et al. 2002). We transplanted the socially polymorphic sweat bee Lasioglossum calceatum from a non-eusocial population at Inverness, in the far north of the UK, 800 km south to a predominantly eusocial population on the University of Sussex campus, in the far south of the UK (Fig. 1). Most native Sussex bees exhibited eusociality, whereas nine of ten transplanted Inverness bees and their offspring exhibited solitary behaviour. Bearing in mind that the sample size is small, we do not wish to overemphasise these precise figures, but our best estimate is that 10% of Inverness bees are capable of expressing eusociality. Our results provide the first field-based experimental evidence that inter-population differences in social phenotype might predominantly reflect genetic differentiation, and provide genetic confirmation that L. calceatum is truly eusocial in the southern UK.
In nine of ten cases, neither spring-transplanted foundresses nor their offspring showed evidence of social plasticity: spring-transplanted foundresses provisioned large B1 female offspring that did not attempt to become workers. By contrast, native foundresses produced small B1 females that typically became workers (Fig. 4a, b). We confirmed that offspring of transplanted foundresses did not enter hibernation simply because their mothers had died (e.g. Packer 1990; Field et al. 2010), since transplanted foundresses were still alive in seven of ten nests at the time of offspring emergence.
One possibility, however, is that because spring-transplanted foundresses developed and overwintered at the Inverness source site, solitary behaviour represents plasticity in response to cues experienced by the foundress prior to transplantation (Thibert-Plante and Hendry 2011). Maternal effects may then influence offspring social phenotype, for example through nutrition provided by mothers (e.g. Brand and Chapuisat 2012; Kapheim et al. 2015b; Berens et al. 2015). However, relatively large B1 females can still become workers in other socially polymorphic sweat bees (Field et al. 2010), and small B1 females can enter hibernation if they emerge late in the season (Hirata and Higashi 2008). This suggests that in species exhibiting plasticity, any nutrition-mediated maternal effects can be overridden by environmental cues experienced by emerging offspring. Furthermore, although we could not be certain of social phenotype, two autumn-transplanted foundresses successfully founded nests that did not become social: one produced two B1 males, and the other a single female that provisioned once before disappearing. Neither scenario was observed among native nests, 28 out of 29 of which became eusocial. These foundresses experienced overwintering conditions at Sussex, yet neither nest became social as expected if social phenotype was plastic, which together with the spring-transplanted nest that became social hints that overwintering conditions alone are unlikely to explain our results. We note the possibility that the male-producing transplanted foundress had not mated prior to capture in the previous autumn.
Another possibility is that emerging later than most native B1 females may have increased the propensity for B1 females of transplanted foundresses to enter hibernation instead of becoming workers (Field et al. 2010; Fig. 5a). Nevertheless, they still emerged earlier in the season than almost all native B1 workers in two previous years (Fig. 5b; Davison and Field 2016), and our analysis showed that neither temperature experienced by foundresses during provisioning nor offspring emergence date successfully explained social phenotype. Moreover, although spring-transplanted foundresses tended to begin provisioning later than Sussex foundresses in this study, four spring-transplanted foundresses that did not produce workers still began provisioning before the latest-provisioning eusocial foundresses in 2012 and 2013 (Fig. 5b). Together this suggests that date of offspring emergence per se might not be a critical factor influencing the social phenotype of L. calceatum. However, we cannot discount the possibility that other unmeasured cues correlated with later foundress provisioning/offspring emergence could have influenced social phenotype.
Plasticity and its loss
Our results show that although most were solitary, transplanted Inverness bees can still express eusociality (see also Plateaux-Quénu et al. 2000): all three B1 female offspring of one spring-transplanted foundress began provisioning the natal nest, behaviour never previously observed at Inverness (Davison and Field 2016). Moreover, once these offspring had emerged, the foundress did not leave the nest, thus expressing the same behaviour as eusocial foundresses native to the Sussex site; and two of the three provisioning offspring were among the smallest produced by transplanted foundresses. Our limited data hint that sociality expressed by Inverness bees might be inefficient: despite the nest containing three provisioning B1 females (mean for native nests = 3.1 ± 0.33), productivity at this nest was lower than at any native nest that successfully produced B2 offspring (Fig. 3).
Phenotypic plasticity can be lost via genetic drift and subsequent genetic assimilation once environmental conditions become predictable (Masel et al. 2007; Pfennig et al. 2010), and when circumstances in which the alternative phenotype is expressed no longer arise (Sikkink et al. 2014; Smith et al. 2015; Cini et al. 2015). At Inverness, B1 females always enter hibernation, whereas at Sussex they may either become workers or enter hibernation (Davison and Field 2016). Plasticity could be lost in solitary populations where emerging offspring only ever receive cues associated with entering hibernation, such as reaching adulthood late in the season (Hirata and Higashi 2008; Field et al. 2010) or mating soon after reaching adulthood (Yanega 1989, 1997; but see Lucas and Field 2013). Therefore, at Inverness, loci regulating eusocial behaviour will not be exposed to selection because female offspring always enter hibernation. This could lead to genetic changes in the response threshold at which eusociality is expressed, and to its eventual loss from the population (Abouheif and Wray 2002; Suzuki and Nijhout 2006; Sikkink et al. 2014). Through this process, for example, the threshold at which bees from Inverness express eusociality might be higher than for native Sussex bees. In the UK, eusociality in sweat bees is restricted to the south (Soro et al. 2010; Falk 2015; Davison and Field 2016), and it is possible that L. calceatum from our Inverness population might exhibit greater plasticity if transplanted further south in Europe to sites where environmental cues for sociality are more extreme (Sikkink et al. 2014).
Local adaptation requires mechanisms that minimise gene flow between eusocial and solitary populations (Lenormand 2002). Without physical barriers to gene flow, one possibility could be differences in the timing of offspring production (Soucy and Danforth 2002; Quintero et al. 2014; Weis 2015). In sympatry, eusocial nests produce reproductive offspring in the second brood, later than the first brood reproductives produced in solitary nests. However, assortative mating may not occur because the first brood in eusocial nests often contains males, together with some females which may mate and enter hibernation without becoming workers (Plateaux-Quénu 1992; Davison and Field 2016; PJD, personal observation).
Eusociality in L. calceatum
We confirmed that native L. calceatum exhibits eusociality at Sussex. Relatedness among B2 female brood within nests was high (r = 0.74), and foundresses surviving to the end of the season tended to monopolise reproduction. In common with other eusocial sweat bees, we also found no evidence that Sussex foundresses mated multiply (Crozier et al. 1987; Packer and Owen 1994; Mueller et al. 1994; Field et al. 2010, but see Soro et al. 2009), consistent with the hypothesis that monandry might help to facilitate the evolution of eusociality (Boomsma 2007; Hughes et al. 2008). Our result contrasts with a recent study of H. scabiosae Rossi, where relatedness among B2 females was considerably lower due to high rates of foundress turnover and frequent drifting between nests (Brand and Chapuisat 2016). We were unable to sample workers comprehensively; however, we did detect at least one likely case of drifting in which an alien B1 female produced a B2 female offspring. We documented no cases of natal workers laying B2 female brood, consistent with the idea that B1 females will reproduce in the presence of the foundress only if she is not their own mother (Paxton et al. 2002).
Conclusion
The possibility that differences in social phenotype between eusocial and solitary populations of L. calceatum primarily reflect genetic differentiation will be of special interest for future studies investigating the genomics of sociality (e.g. Kocher et al. 2013). Few studies have examined the extent to which social polymorphism has promoted population differentiation (e.g. see Soucy and Danforth 2002; Zayed and Packer 2002; Soro et al. 2010), or considered whether polymorphism could facilitate ecological speciation (Rundle and Nosil 2005; Thibert-Plante and Hendry 2011). It would be interesting to transplant bees from a less northerly solitary population, where selection for plasticity may have persisted and bees may reveal a lower threshold for the expression of eusociality. Furthermore, because both eusocial and solitary nesting has been recorded at Sussex, and eusocial foundresses routinely pass through a solitary phase in spring prior to worker emergence, bees from Sussex may be more predisposed to exhibit plasticity if transplanted to Inverness. In general, the cornucopia of social variation exhibited by sweat bees demands that species are studied in detail throughout their geographic range, and in a variety of environmental contexts (Wcislo and Danforth 1997; Wcislo 1997).
References
Abouheif E, Wray GA (2002) Evolution of the gene network underlying wing polyphenism in ants. Science 297:249–252. https://doi.org/10.1126/science.1071468
Allison P (2012) When can you safely ignore multicollinearity? Statistical Horizons http://statisticalhorizons.com/multicollinearity. Accessed 29 November 2015
Baglione V, Canestrari D, Marcos JM, Griesser M, Ekman J (2002) History, environment and social behaviour: experimentally induced cooperative breeding in the carrion crow. P Roy Soc Lond B Bio 269:1247–1251. https://doi.org/10.1098/rspb.2002.2016
Berens AJ, Hunt JH, Toth AL (2015) Comparative transcriptomics of convergent evolution: different genes but conserved pathways underlie caste phenotypes across lineages of eusocial insects. Mol Biol Evol 32:690–703. https://doi.org/10.1093/molbev/msu330
Boomsma JJ (2007) Kin selection versus sexual selection: why the ends do not meet. Curr Biol 17:673–683. https://doi.org/10.1016/j.cub.2007.06.033
Brady SG, Sipes S, Pearson A, Danforth BN (2006) Recent and simultaneous origins of eusociality in halictid bees. P Roy Soc Lond B Bio 273:1643–1649. https://doi.org/10.1098/rspb.2006.3496
Brand N, Chapuisat M (2012) Born to be bee, fed to be worker? The caste system of a primitively eusocial insect. Front Zool 9:35. https://doi.org/10.1186/1742-9994-9-35
Brand N, Chapuisat M (2016) Low relatedness and frequent inter-nest movements in a eusocial sweat bee. Insect Soc 63:249–256. https://doi.org/10.1007/s00040-015-0460-0
Cini A, Patalano S, Segonds-Pichon A, Busby GB, Cervo R, Sumner S (2015) Social parasitism and the molecular basis of phenotypic evolution. Front Genet 6:32. https://doi.org/10.3389/fgene.2015.00032
Chapuisat M (2010) Evolution: plastic sociality in a sweat bee. Curr Biol 20:R977–R979. https://doi.org/10.1016/j.cub.2010.10.033
Crawley MJ (2013) The R book, 2nd edn. Wiley, Singapore
Cronin AL (2001) Social flexibility in a primitively social allodapine bee (Hymenoptera: Apidae): results of a translocation experiment. Oikos 94:337–343. https://doi.org/10.1034/j.1600-0706.2001.940214.x
Crozier RH, Smith BH, Crozier YC (1987) Relatedness and population structure of the primitively eusocial bee Lasioglossum zephyrum (Hymenoptera: Halictidae) in Kansas. Evolution 41:902–910
Danforth BN (2002) Evolution of sociality in a primitively eusocial lineage of bees. Proc Nat Acad Sci USA 99:286–290. https://doi.org/10.1073/pnas.012387999
Danforth BN, Conway L, Ji S (2003) Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees (Hymenoptera: Halictidae). Syst Biol 52:23–36
Davison PJ, Field J (2016) Social polymorphism in the sweat bee Lasioglossum (Evylaeus) calceatum. Insect Soc 63:327–338. https://doi.org/10.1007/s00040-016-0473-3
Davison PJ, Field J (2017) Season length, body size and social polymorphism: size clines but not saw tooth clines in sweat bees. Ecol Entomol 42:768–776. https://doi.org/10.1111/een.12448
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genet Resour 4:359–361. https://doi.org/10.1007/s12686-011-9548-7
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Falk S (2015) Field guide to the bees of Great Britain and Ireland. Bloomsbury, London and New York
Field J, Paxton RJ, Soro A, Bridge C (2010) Cryptic plasticity underlies a major evolutionary transition. Curr Biol 20:2028–2031. https://doi.org/10.1016/j.cub.2010.10.020
Field J, Paxton R, Soro A, Craze P, Bridge C (2012) Body size, demography and foraging in a socially plastic sweat bee: a common garden experiment. Behav Ecol Sociobiol 66:743–756. https://doi.org/10.1007/s00265-012-1322-7
Fox J, Weisberg S (2011) An {R} companion to applied regression, Second edn. Sage, Thousand Oaks CA
Gibbs J, Brady SG, Kanda K, Danforth BN (2012) Phylogeny of halictine bees supports a shared origin of eusociality for Halictus and Lasioglossum (Apoidea: Anthophila: Halictidae). Mol Phylogenet Evol 65:926–939. https://doi.org/10.1016/j.ympev.2012.08.013
Goodnight KF, Queller DC (1999) Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol Ecol 8:1231–1234
Hay SI, Tatem AJ, Graham AJ, Goetz SJ, Rogers DJ (2006) Global environmental data for mapping infectious disease distribution. In: Hay SI, Graham AJ, Rogers DJ (eds) Advances in parasitology Vol. 62. Academic Press, Great Britain, pp 37–71
Hirata M, Higashi S (2008) Degree-day accumulation controlling allopatric and sympatric variations in the sociality of sweat bees, Lasioglossum (Evylaeus) baleicum (Hymenoptera: Halictidae). Behav Ecol Sociobiol 62:1239–1247. https://doi.org/10.1007/s00265-008-0552-1
Hughes WHO, Oldroyd BP, Beekman M, Ratnieks FWL (2008) Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320:1213–1216. https://doi.org/10.1126/science.1156108
Kocher SD, Li C, Yang W, Tan H, Yi SV, Yang X, Hoekstra H, Zhang G, Pierce N, Yu DW (2013) The draft genome of a socially polymorphic halictid bee, Lasioglossum albipes. Genome Biol 14:R142. https://doi.org/10.1186/gb-2013-14-12-r142
Kapheim KM, Smith AR, Ihle KE, Amdam GV, Nonacs P, Wcislo WT (2012) Physiological variation as a mechanism for developmental caste-biasing in a facultatively eusocial sweat bee. P Roy Soc Lond B Bio 279:1437–1446. https://doi.org/10.1098/rspb.2011.1652
Kapheim KM, Pan H, Li C et al (2015a) Genomic signatures of evolutionary transitions from solitary to group living. Science 348:1139–1143. https://doi.org/10.1126/science.aaa4788
Kapheim KM, Nonacs P, Smith AR, Wayne RK, Wcislo WT (2015b) Kinship, parental manipulation and evolutionary origins of eusociality. P Roy Soc Lond B Bio 282:20142886. https://doi.org/10.1098/rspb.2014.2886
Lenormand T (2002) Gene flow and the limits to natural selection. Trends Ecol Evol 17:183–189. https://doi.org/10.1016/S0169-5347(02)02497-7
Lucas ER, Field J (2013) Caste determination through mating in primitively eusocial societies. J Theor Biol 335:31–39. https://doi.org/10.1016/j.jtbi.2013.05.032
Masel J, King OD, Maughan H (2007) The loss of adaptive plasticity during long periods of environmental stasis. Am Nat 169:38–46. https://doi.org/10.1086/510212
Mueller UG, Eickwort GC, Aquadro CF (1994) DNA fingerprinting analysis of parent-offspring conflict in a bee. Proc Nat Acad Sci USA 91:5143–5147
Nicholls JA, Double MC, Rowell DM, Magrath RD (2000) The evolution of cooperative and pair breeding in thornbills Acanthiza (Pardalotida). J Avian Biol 31:165–176. https://doi.org/10.1034/j.1600-048X.2000.310208.x
Packer L (1990) Solitary and eusocial nests in a population of Augochlorella striata (Provancher) (Hymenoptera; Halictidae) at the northern edge of its range. Behav Ecol Sociobiol 27:339–344
Packer L, Knerer G (1985) Social evolution and its correlates in bees of the subgenus Evylaeus. Behav Ecol Sociobiol 17:143–149
Packer L, Owen RE (1994) Relatedness and sex ratio in a primitively eusocial halictine bee. Behav Ecol Sociobiol 34:1–10
Parsons PJ, Couchoux C, Horsburgh GJ, Dawson DA, Field J (2017) Identification of 24 new microsatellite loci in the sweat bee Lasioglossum malachurum (Hymenoptera: Halictidae). BMC Res Notes 10:753. https://doi.org/10.1186/s13104-017-3089-4
Paxton RJ, Ayasse M, Field J, Soro A (2002) Complex sociogenetic organization and reproductive skew in a primitively eusocial sweat bee, Lasioglossum malachurum, as revealed by microsatellites. Mol Ecol 11:2405–2416
Pesenko YA, Banaszak J, Radchenko VG, Cierzniak T (2000) Bees of the family Halictidae (excluding Sphecodes) of Poland: taxonomy, ecology, bionomics. Wydawnictwo Uczelniane Wyższej Szkoły Pedagogicznej w Bydgoszczy, Bydgoszcz
Pfennig DW, Wund MA, Snell-Rood E, Cruickshank T, Schlichting CD, Moczek AP (2010) Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25:459–467. https://doi.org/10.1016/j.tree.2010.05.006
Plateaux-Quénu C (1983) Le volume d’un pain d’abeille influence-t-il le sexe de l’oeuf pondu sur lui? Etude experimental portant sur la premiere couvee d'Evylaeus calceatus (Scop.) (Hym., Halictinae). Annales des Sciences Naturelles Zoologie 13e(serie 5):41–52
Plateaux-Quénu C (1992) Comparative biological data in two closely related eusocial species Evylaeus calceatus (Scop.) and Evylaeus albipes (F.) (Hym., Halictinae). Insect Soc 39:351–364
Plateaux-Quénu C, Plateaux L, Packer L (2000) Population-typical behaviours are retained when eusocial and non-eusocial forms of Evylaeus ablbipes (F.) (Hymenoptera, Halictidae) are reared simultaneously in the laboratory. Insect Soc 47:263–270. https://doi.org/10.1007/PL00001713
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Queller DC, Goodnight KF (1989) Estimating relatedness using genetic markers. Evolution 43:258–275
Quintero I, González-Caro S, Zalamea P-C, Cadena CD (2014) Asynchrony of seasons: genetic differentiation associated with geographic variation in climatic seasonality and reproductive phenology. Am Nat 184:352–363. https://doi.org/10.1086/677261
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Heredity 86:248–249
Rousset F (2008) Genepop'007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Resources 8:103–106. https://doi.org/10.1111/j.1471-8286.2007.01931.x
Rehan SM, Toth AL (2015) Climbing the social ladder: the molecular evolution of sociality. Trends Ecol Evol 30:426–433. https://doi.org/10.1016/j.tree.2015.05.004
Richards MH, Packer L (1994) Trophic aspects of caste determination in Halictus ligatus, a primitively eusocial sweat bee. Behav Ecol Sociobiol 34:385–391
Richards MH, Onuferko TM, Rehan SM (2015) Phenological, but not social, variation associated with climate differences in a eusocial sweat bee, Halictus ligatus, nesting in southern Ontario. J Hym Res 43:19–44. https://doi.org/10.3897/JHR.43.8756
Rundle H, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352. https://doi.org/10.1111/j.1461-0248.2004.00715.x
Sakagami S, Michener CD (1962) The nest architecture of the sweat bees (Halictinae): a comparative study of behavior. University of Kansas Press, Lawrence
Sakagami SF, Fukuda H (1972) Life of a Japanese eusocial halictine bee Lasioglossum duplex, out of brood rearing season (Hymenoptera, Halictidae). Insect Soc 19:137–152
Sakagami SF, Munakata M (1972) Distribution and bionomics of a transpalearctic eusocial halictine bee, Lasioglossum (Evylaeus) calceatum, in Northern Japan, with reference to its solitary life cycle at high altitude. Hokkaido Univ Fac Sci J Ser Vi Zool 18:411–439
Schwarz MP, Richards MH, Danforth BN (2007) Changing paradigms in insect social evolution: insights from halictine and allodapine bees. Annu Rev Entomol 52:127–150. https://doi.org/10.1146/annurev.ento.51.110104.150950
Sikkink KL, Reynolds RM, Ituarte CM, Cresko WA, Phillips PC (2014) Rapid evolution of phenotypic plasticity and shifting thresholds of genetic assimilation in the nematode Caenorhabditis remanei. G3 (Bethesda) 4:1103–1112. https://doi.org/10.1534/g3.114.010553
Smith CR, Cahan SH, Kemena C et al (2015) How do genomes create novel phenotypes? Insights from the loss of the worker caste in ant social parasites. Mol Biol Evol 32:2919–1931. https://doi.org/10.1093/molbev/msv165
Soro A, Ayasse M, Zobel MU, Paxton RJ (2009) Complex sociogenetic organization and the origin of unrelated workers in a eusocial sweat bee, Lasioglossum malachurum. Insect Soc 56:55–63. https://doi.org/10.1007/s00040-008-1037-y
Soro A, Field J, Bridge C, Cardinal SC, Paxton RJ (2010) Genetic differentiation across the social transition in a socially polymorphic sweat bee, Halictus rubicundus. Mol Ecol 19:3351–3363. https://doi.org/10.1111/j.1365-294X.2010.04753.x
Soucy SL, Danforth BN (2002) Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Evolution 56:330–341
Storey JD (2002) A direct approach to false discovery rates. J R Statist Soc B 64:479–498. https://doi.org/10.1111/1467-9868.00346
Suzuki Y, Nijhout F (2006) Evolution of polyphenism by genetic accommodation. Science 311:650–652. https://doi.org/10.1126/science.1118888
Thibert-Plante X, Hendry AP (2011) The consequences of phenotypic plasticity for ecological speciation. J Evol Biol 24:326–342. https://doi.org/10.1111/j.1420-9101.2010.02169.x
Verhoeven KJF, Simonsen KL, McIntyre LM (2005) Implementing false discovery rate control: increasing your power. Oikos 108:643–647. https://doi.org/10.1111/j.0030-1299.2005.13727.x
Wcislo WT (1997) Behavioral environments of the sweat bees. In: Choe JC, Crespi B (eds) Social behaviour in insects and arachnids. Cambridge University Press, Cambridge, pp 316–332
Wcislo WT, Danforth BN (1997) Secondarily solitary: the evolutionary loss of social behavior. Trends Ecol Evol 12:468–474
Weis AE (2015) On the potential strength and consequences for nonrandom gene flow caused by local adaptation in flowering time. J Evol Biol 28:699–714. https://doi.org/10.1111/jeb.12612
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Yanega D (1989) Caste determination and differential diapause within the first brood of Halictus rubicundus in New York (Hymenoptera: Halictidae). Behav Ecol Sociobiol 24:97–107
Yanega D (1997) Halictine demography and sociality. In: Choe JC, Crespi B (eds) Social behavior in insects and arachnids. Cambridge University Press, Cambridge pp, pp 293–315
Zayed A, Packer L (2002) Genetic differentiation across a behavioural boundary in a primitively eusocial bee, Halictus poeyi Lepeletier (Hymenoptera, Halictidae). Insect Soc 49:282–288. https://doi.org/10.1007/s00040-002-8315-x
Acknowledgements
We thank Paul Parsons for performing genotyping and help with the implementation of STRUCTURE, Rebecca Fuller for assisting with behavioural observations, and three anonymous reviewers for commenting on the manuscript.
Funding
This work formed part of a studentship (1119965) awarded to PJD funded by the Natural Environment Research Council and the University of Sussex, supervised by JF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Heinze
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Davison, P.J., Field, J. Limited social plasticity in the socially polymorphic sweat bee Lasioglossum calceatum. Behav Ecol Sociobiol 72, 56 (2018). https://doi.org/10.1007/s00265-018-2475-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2475-9