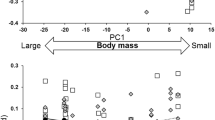
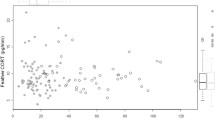
Abstract
Despite growing appreciation of the importance of considering a pace-of-life syndrome (POLS) perspective to understand how animals interact with their environment, studies relating behavior to life history under altered environmental conditions are still rare. By means of a comparative analysis of flight initiation distances (i.e., the distance at which an animal takes flight when a human being is approaching) across > 300 bird species distributed worldwide, we document here the existence of a POLS predicted by theory where slow-lived species tend to be more risk-averse than fast-lived species. This syndrome largely emerges from the influence of body mass, and is highly dependent on the environmental context. Accordingly, the POLS structure vanishes in urbanized environments due to slow-lived species adjusting their flight distances based on the perception of risk. While it is unclear whether changes in POLS reflect plastic and/or evolutionary adjustments, our findings highlight the need to integrate behavior into life history theory to fully understand how animals tolerate human-induced environmental changes.
Significance statement
Animals can often respond to changing environmental conditions by adjusting their behavior. However, the degree to which different species can modify their behavior depends on their life history strategy and on the environmental context. Species-specific perception of risk is a conspicuous example of adjustable behavior tightly associated with life history strategy. While there is a general tendency of higher risk aversion in rural than city-dwelling birds, it is dependent on the species’ life history strategy. Slow-lived species are more prone to adjust their flight initiation distances based on the perception of risk, allowing humans to approach closer in urban than rural environments. Behavior must therefore be taken into account together with life history to reliably assess species’ vulnerability at the face of ongoing environmental change.





Similar content being viewed by others
References
Adler PB, Salguero-gómez R, Compagnoni A, Hsu JH, Ray-Mukherjee J, Mbeau-Ache C, Franco M (2014) Functional traits explain variation in plant life history strategies. Proc Natl Acad Sci USA 111:740–745
Blumstein DT (2006) Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim Behav 71:389–399
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Bogert C (1949) Thermoregulation in reptiles, a factor in evolution. Evolution 3:195–211
Carrete M, Tella JL (2011) Inter-individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS One 6:e18859
Caswell H (2000) Prospective and retrospective perturbation analyses: their roles in conservation biology. Ecology 81:619–627
Charmantier A, Demeyrier V, Lambrechts M, Perret S, Grégoire A (2017) Urbanization is associated with divergence in pace-of-life in great tits. Front Ecol Evol 5:53
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Estrada A, Morales-Castilla I, Caplat P, Early R (2016) Usefulness of species traits in predicting range shifts. Trends Ecol Evol 31:190–203
Evans KL, Newton J, Gaston KJ, Sharp SP, McGowan A, Hatchwell BJ (2012) Colonisation of urban environments is associated with reduced migratory behaviour, facilitating divergence from ancestral populations. Oikos 121:634–640
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160:712–726
Gonzalez-Voyer A, von Hardenberg A (2014) An introduction to phylogenetic path analysis. In: Garamszegi LZ (ed) Modern phylogenetic comparative methods and their application in evolutionary biology. Springer-Verlag, Berlin, pp 201–229
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86
Greenberg R (2003) The role of neophobia and neophilia in the development of innovative behaviour of birds. In: Reader SM, Laland KN (eds) Animal innovation. Oxford University Press, Oxford, pp 176–196
Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22
Hadfield JD, Nakagawa S (2010) General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J Evol Biol 23:494–508
Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD (2010) Corticosterone, testosterone and life-history strategies of birds. Proc Biol Sci 277:3203–3212
Hediger H (1934) Zur biologie und Psychologie der Flucht bei Tieren. Biol ZBL 54:21–40
Hemmingsen A (1951) The relation of shyness (flushing distance) to body size. Spolia Zool Musei Hauniensis 11:74–76
Hille SM, Cooper CB (2014) Elevational trends in life histories: revising the pace-of-life framework. Biol Rev 90:204–213
Housworth EA, Martins P, Lynch M (2004) The phylogenetic mixed model. Am Nat 163:84–96
Huey RB, Hertz PE, Sinervo B (2003) Behavioral drive versus behavioral inertia in evolution: a null model approach. Am Nat 161:357–366
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO (2012) The global diversity of birds in space and time. Nature 491:444–448
Kark S, Iwaniuk A, Schalimtzek A, Banker E (2007) Living in the city: can anyone become an “urban exploiter”? J Biogeogr 34:638–651
Klopfer P (1962) Behavioral aspects of ecology. Prentice-Hall International Inc., London
Koolhaas JM, Korte SM, de Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23:925–935
Lee WY, Lee SI, Choe JC, Jablonski PG (2011) Wild birds recognize individual humans: experiments on magpies, Pica pica. Anim Cogn 14:817–825
Levey DJ, Londoño GA, Ungvari-Martin J, Hiersoux MR, Jankowski JE, Poulsen JR, Stracey CM, Robinson SK (2009) Urban mockingbirds quickly learn to identify individual humans. Proc Natl Acad Sci USA 106:8959–8962
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549
Maklakov AA, Immler S, Gonzalez-Voyer A, Rönn J, Kolm N (2011) Brains and the city: big-brained passerine birds succeed in urban environments. Biol Lett 7:730–732
Martin TE, Martin PR, Olson CR, Heidinger BJ, Fontaine JJ (2000) Parental care and clutch sizes in north and south American birds. Science 287:1482–1485
Mayr E (1965) The nature of colonising birds. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic press, New York, pp 29–43
Møller AP (1994) Sexual selection and the barn swallow. Oxford University Press
Møller AP (2008) Flight distance of urban birds, predation, and selection for urban life. Behav Ecol Sociobiol 63:63–75
Møller AP (2010) Interspecific variation in fear responses predicts urbanization in birds. Behav Ecol 21:365–371
Møller AP (2015) Birds. In: Cooper WJ, Blumstein D (eds) Escaping from predators: an integrative view of escape decisions and refuge use. Cambridge University Press, Cambridge, pp 88–112
Møller AP, Diaz M, Flensted-Jensen E, Grim T, Ibañez-Alamo JD, Jokimäki J, Mänd R, Markó G, Tryjanowski P (2015) Urbanized birds have superior establishment success in novel environments. Oecologia 178:943–950
Møller AP, Erritzøe J (2014) Predator-prey interactions, flight initiation distance and brain size. J Evol Biol 27:34–42
Møller AP, Grim T, Ibáñez-Álamo JD, Markó G, Tryjanowski P (2013) Change in flight initiation distance between urban and rural habitats following a cold winter. Behav Ecol 24:1211–1217
Møller AP, Liang W (2013) Tropical birds take small risks. Behav Ecol 24:267–272
Møller AP, Garamszegi LZ (2012) Between individual variation in risk taking behavior and its life history consequences. Behav Ecol 23:843–853
Oli MK (2004) The fast-slow continuum and mammalian life-history patterns: an empirical evaluation. Basic Appl Ecol 5:449–463
Oli MK, Dobson FS (2003) The relative importance of life-history variables to population growth rate in mammals: Cole’s prediction revisited. Am Nat 161:422–440
Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W (2013). Caper: comparative analyses of Phylogenetics and evolution in R. R package version 0.5.2. https://CRAN.R-project.org/package=caper
Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L (2009) Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim Behav 78:1001–1010
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Perals D, Griffin AS, Bartomeus I, Sol D (2017) Revisiting the open-field test: what does it really tell us about animal personality? Anim Behav 123:69–79
Price T, Schulter D (1991) On the low heritability of life-history traits. Evolution 45:853–861
Price TD, Qvarnstrom A, Irwin DE (2003) The role of phenotypic plasticity in driving genetic evolution. Proc R Soc Lond B 270:1433–1440
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos Trans R Soc Lond B Biol Sci 365:4051–4063
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse N (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Revell LJ (2009) Size-correction and principal components for interspecific comparative studies. Evolution 63:3258–3268
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Saether B-E (1988) Pattern of covariation between life-history traits of european birds. Nature 331:616–617
Saether B-E, Engen S (2003) Routes to extinction. In: Blackburn TM, Gaston KJ (eds) Macroecology - concepts and consequences. Cambridge University Press, pp 218–236
Salguero Gomez R, Jones O, Archer R et al (2016) COMADRE: a global database of animal demography. J Anim Ecol 85:371–384
Sayol F, Maspons J, Lapiedra O, Iwaniuk AN, Székely T, Sol D (2016) Environmental variation and the evolution of large brains in birds. Nat Commun 7:13971
Shipley B (2013) The AIC model selection method applied to path analytic models compared using a d-separation test. Ecology 94:560–564
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15:278–289
Sih A, Del Giudice M (2012) Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc Lond B Biol Sci 367:2762–2772
Sol D (2009a) The cognitive-buffer hypothesis for the evolution of large brains. In: Dukas R, Ratcliffe RM (eds) . Cogn Ecol. Chicago University Press, Chicago, pp 111–134
Sol D (2009b) Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol Lett 5:130–133
Sol D, Bartomeus I, Griffin AS (2012a) The paradox of invasion in birds: competitive superiority or ecological opportunismz? Oecologia 169:553–564
Sol D, González-Lagos C, Moreira D, Maspons J, Lapiedra O (2014) Urbanisation tolerance and the loss of avian diversity. Ecol Lett 17:942–950
Sol D, Griffin AS, Bartomeus I, Boyce H (2011) Exploring or avoiding novel food resources ? The novelty conflict in an invasive bird. PLoS One 6:e19535
Sol D, Lapiedra O, González-lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112
Sol D, Maspons J (2016) Life history, behaviour and invasion success. In: Weis J, Sol D (eds) Biological invasions and animal behaviour. Cambridge University Press, Cambridge, pp 63–82
Sol D, Maspons J, Vall-Llosera M, Bartomeus I, García-Peña G, Piñol J, Freckleton RP (2012b) Unravelling the life history of successful invaders. Science 337:580–583
Sol D, Sayol F, Ducatez S, Lefebvre L (2016) The life-history basis of behavioural innovations. Philos Trans R Soc Lond B Biol Sci 371:20150187
Stearns SC (1983) The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos 41:173–187
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stearns SC (2000) Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87:476–486
Tieleman B, Williams JB, Ricklefs RE, Klasing KC (2005) Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc R Soc Lond B 272:1715–1720
van Schaik CP, Deaner RO (2003) Life history and cognitive evolution in primates. In: de Waal FBM, Tyack PL (eds) Animal social complexity. Harvard University Press, Cambridge, pp 5–25
Verbeek ME, Drent PJ, Wiepkema PR (1994) Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav 48:1113–1121
von Hardenberg A, Gonzalez-Voyer A (2013) Disentangling evolutionary cause-effect relationships with phylogenetic confirmatory path analysis. Evolution 67:378–387
Wolf M, van Doorn GS, Leimar O, Weissing FJ (2007) Life-history trade-offs favour the evolution of animal personalities. Nature 447:581–584
Acknowledgements
We wish to thank Melanie Dammhahn, Denis Réale, Niels Dingemanse, and Petri Niemela for kindly inviting us to the workshop “Towards a general theory of the pace-of-life syndrome” funded by Volkswagen Foundation—VolkswagenStiftung, which stimulated the discussions that prompted the present study, and Niels Dingemanse and three reviewers for insightful comments in previous versions of the manuscript.
Funding
DS was supported by the project CGL2013-47448-P from the Spanish Government, AGV by project 2013–4834 from the Swedish Research Council and project IA201716 from PAPIIT, UNAM, IMC by the Fonds de Recherches du Quebec—Nature et Technologies (FQRNT) programme and by Harvard University, and LZG was supported by funds from The Ministry of Economy and Competitiveness (Spain) (CGL2015-70639-P) and The National Research, Development and Innovation Office (Hungary) (K-115970).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
The study was conducted in accordance with the current laws in all visited countries. We recorded FID by approaching birds in the field until they flew away. However, this activity is similar to what birds experience when people walk around in urban environments, and there are no known negative effects of such activities on the behavior of birds.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by N. Dingemanse
This article is a contribution to the Topical Collection Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life-history – Guest Editors: Melanie Dammhahn, Niels J. Dingemanse, Petri T. Niemelä, Denis Réale
Rights and permissions
About this article
Cite this article
Sol, D., Maspons, J., Gonzalez-Voyer, A. et al. Risk-taking behavior, urbanization and the pace of life in birds. Behav Ecol Sociobiol 72, 59 (2018). https://doi.org/10.1007/s00265-018-2463-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2463-0