Abstract
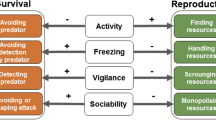
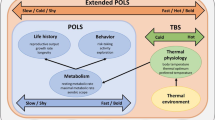
Correlations between behavioral, physiological, and morphological traits linked to life history have been given the label “pace-of-life syndrome” (POLS), hypothesized to arise through variation in the resolution of a trade-off between present and future reproduction. However, other trade-offs over energy allocation may also have effects and influence the present-future trade-off. We analyzed an optimality model of basal metabolic rate (BMR) across variation in food availability and two types of mortality. The model contained three major features: (1) feedback between activity and energy acquisition, (2) links between BMR and the use of energy for other traits, and (3) allocation trade-offs between BMR and all other traits, between activity and defense, and between defense against activity-related risk and activity-independent risk. The model produced an intermediate optimal BMR that was usually highest at an intermediate level of food availability. Food availability and both types of mortality risk interacted to influence the exact value of optimal BMR. Trait correlations expected in the POLS existed under some environmental conditions, but these correlations flipped sign under different conditions and were not always strong. Our model reproduces trait correlations consistent with the POLS, but also generated a “sloppy” syndrome with considerable non-POLS-like variation. In addition, among-individual, non-adaptive variation in BMR produced adjustments of the other traits. These fit a best-of-a-bad job strategy, and the adjustments further weakened trait correlations. The results emphasize that variation in resources and mortality risk creates a diversity of correlation structures. This complexity means the POLS is likely to be a variable construct.
Significance statement
Many attributes important for reproduction and survival are associated. Such associations may arise through common physiological processes and correlated selection. We modeled metabolic rate within a system in which foraging behavior both depended on and mediated the acquisition of resources necessary for metabolism, while energy was allocated among multiple attributes. Variation in several environmental variables (food availability and two types of mortality risk) influenced basal metabolic rate, activity, and defenses against mortality risk. This variation affected the correlations between the traits in complex ways. When basal metabolic rate was non-optimal, evolution of the allocation of energy to other traits partially compensated, but this further eroded consistent trait correlations. Our results indicate that complexity in how energy is acquired and used can potentially disrupt trait correlations normally associated with the pace-of-life syndrome.






Similar content being viewed by others
Change history
23 April 2018
Equation 3 of the original published version of this article was incorrect. Correct presentation is given below.
References
Adler PB, Salguero-Gómez R, Compagnoni A, Hsu JS, Ray-Mukherjee J, Mbeau-Ache C, Franco M (2014) Functional traits explain variation in plant life history strategies. P Natl Acad Sci USA 111:740–745
Adriaenssens B, Johnsson JI (2011) Shy trout grow faster: exploring links between personality and fitness-related traits in the wild. Behav Ecol 22:135–143
Anholt BR, Werner EE (1995) Interaction between food availability and predation mortality mediated by adaptive behavior. Ecology 76:2230–2234
Arendt JD, Reznick DN (2005) Evolution of juvenile growth rates in female guppies (Poecilia reticulata): predator regime or resource level? Proc R Soc Lond B 272:333–337
Barber I, Dingemanse NJ (2010) Parasitism and the evolutionary ecology of animal personality. Philos T Roy Soc B 365:4077–4088
Bauwens D, Diaz-Uriarte R (1997) Covariation of life-history traits in lacertid lizards: a comparative study. Am Nat 149:91–111
Bielby J, Mace GM, Bininda-Emonds ORP, Cardillo M, Gittleman JL, Jones KE, Orme CDL, Purvis A (2007) The fast-slow continuum in mammalian life history: an empirical reevaluation. Am Nat 169:748–757
Biro PA, Abrahams MV, Post JR, Parkinson EA (2004) Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc R Soc Lond B 271:2233–2237
Biro PA, Abrahams MV, Post JR, Parkinson EA (2006) Behavioural trade-offs between growth and mortality explain evolution of submaximal growth rates. J Anim Ecol 75:1165–1171
Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol Evol 25:653–659
Bjørkvoll E, Grøtan V, Aanes S, Sæther BE, Engen S, Aanes R (2012) Stochastic population dynamics and life-history variation in marine fish species. Am Nat 180:372–387
Blumstein DT (2006) Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim Behav 71:389–399
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Burton T, Killen SS, Armstrong JD, Metcalfe NB (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc R Soc Lond B 278:3465–3473
Careau V, Bininda-Emonds ORP, Thomas DW, Réale D, Humphries MM (2009) Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct Ecol 23:150–156
Careau V, Garland Jr. T (2012) Performance, personality, and energetics: correlation, causation, and mechanism. Physiol Biochem Zool 85:543–571
Careau V, Réale D, Humphries MM, Thomas DW (2010) The pace of life under artificial selection: personality, energy expenditure, and longevity are correlated in domestic dogs. Am Nat 175:753–758
Careau V, Thomas D, Humphries MM, Réale D (2008) Energy metabolism and animal personality. Oikos 117:641–653
Careau V, Thomas D, Pelletier F, Turki L, Landry F, Garant D, Réale D (2011) Genetic correlation between resting metabolic rate and exploratory behaviour in deer mice (Peromyscus maniculatus). J Evol Biol 24:2153–2163
Clusella Trullas S, van Wyk JH, Spotila JR (2007) Thermal melanism in ectotherms. J Therm Biol 32:235–245
Daan S, Masman D, Groenewold A (1990) Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am J Phys 259:R333–R340
Dawkins R (1976) The selfish gene, 2nd edn. Oxford University Press, New York City
Derting TL, Compton S (2003) Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus). Physiol Biochem Zool 76:744–752
Dingemanse NJ, Dochtermann NA (2013) Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol 82:39–54
Einum S (2014) Ecological modeling of metabolic rates predicts diverging optima across food abundances. Am Nat 183:410–417
Gaillard JM, Yoccoz NG, Lebreton JD, Bonenfant C, Devillard S, Loison A, Pontier D, Allaine D (2005) Generation time: a reliable metric to measure life-history variation among mammalian populations. Am Nat 166:119–123
Gale BH, Johnson JB, Schaalje GB, Belk MC (2013) Effects of predation environment and food availability on somatic growth in the livebearing fish Brachyrhaphis rhabdophora (Pisces: Poeciliidae). Ecol Evol 3:326–333
Gifford ME, Clay TA, Careau V (2014) Individual (co) variation in standard metabolic rate, feeding rate, and exploratory behavior in wild-caught semiaquatic salamanders. Physiol Biochem Zool 87:384–396
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Goodwin NB, Grant A, Perry AL, Dulvy NK, Reynolds JD (2006) Life history correlates of density-dependent recruitment in marine fishes. Can J Fish Aquat Sci 63:494–509
Harrison KV, Preisser EL (2016) Dropping behavior in the pea aphid (Hemiptera: Aphididae): how does environmental context affect antipredator responses? J Insect Sci 16:1–5
Höjesjö J, Adriaenssens B, Bohlin T, Jönsson C, Hellström I, Johnsson JI (2011) Behavioural syndromes in juvenile brown trout (Salmo trutta); life history, family variation and performance in the wild. Behav Ecol Sociobiol 65:1801–1810
Holling C (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Houston AI (2010) Evolutionary models of metabolism, behaviour and personality. Philos T Roy Soc B 365:3969–3975
Houston AI, McNamara JM (2014) Foraging currencies, metabolism and behavioural routines. J Anim Ecol 83:30–40
Karels TJ, Byrom AE, Boonstra R, Krebs CJ (2000) The interactive effects of food and predators on reproduction and overwinter survival of arctic ground squirrels. J Anim Ecol 69:235–247
Konarzewski M, Diamond J (1995) Evolution of basal metabolic rate and organ masses in laboratory mice. Evolution 49:1239–1248
Konarzewski M, Książek A (2013) Determinants of intra-specific variation in basal metabolic rate. J Comp Physiol B 183:27–41
Krams I, Kivleniece I, Kuusik A, Krama T, Freeberg TM, Mänd R, Vrublevska J, Rantala MJ, Mänd M (2013) Predation selects for low resting metabolic rate and consistent individual differences in anti-predator behavior in a beetle. Acta Ethol 16:163–172
Krams IA, Niemelä PT, Trakimas G, Krams R, Burghardt GM, Krama T, Kuusik A, Mänd M, Rantala MJ, Mänd R, Kekäläinen J, Sirkka I, Luoto S, Kortet R (2017) Metabolic rate associates with, but does not generate covariation between, behaviours in western stutter-trilling crickets, Gryllus integer. Proc R Soc B 284:20162481
Krause ET, Liesenjohann T (2012) Predation pressure and food abundance during early life alter risk-taking behaviour and growth of guppies (Poecilia reticulata). Behaviour 149:1–14
Le Galliard JF, Paquet M, Cisel M, Montes-Poloni L (2013) Personality and the pace-of-life syndrome: variation and selection on exploration, metabolism and locomotor performances. Funct Ecol 27:136–144
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Løvlie H, Immonen E, Gustavsson E, Kazancioğlu E, Arnqvist G (2014) The influence of mitonuclear genetic variation on personality in seed beetles. Proc R Soc B 281:20141039
Martel SI, Riquelme SA, Kalergis AM, Bozinovic F (2014) Dietary effect on immunological energetics in mice. J Comp Physiol B 184:937–944
Martin LB, Kilvitis HJ, Brace AJ, Cooper L, Haussmann MF, Mutati A, Fasanello V, O'Brien S, Ardia DR (2017) Costs of immunity and their role in the range expansion of the house sparrow in Kenya. J Exp Biol 220:2228–2235
Martin LB, Scheuerlein A, Wikelski M (2003) Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond B 270:153–158
Mathot KJ, Dingemanse NJ (2015) Energetics and behavior: unrequited needs and new directions. Trends Ecol Evol 30:199–206
Mathot KJ, Frankenhuis W (2018) Models of pace-of-life syndromes (POLS): a systematic review. Behav Ecol Sociobiol. https://doi.org/10.1007/s00265-018-2459-9
Mathot KJ, Martin K, Kempenaers B, Forstmeier W (2013) Basal metabolic rate can evolve independently of morphological and behavioural traits. Heredity 111:175–181
Mathot KJ, Nicolaus M, Araya-Ajoy YG, Dingemanse NJ, Kempenaers B (2015) Does metabolic rate predict risk-taking behaviour? A field experiment in a wild passerine bird. Funct Ecol 29:239–249
McNab BK (1997) On the utility of uniformity in the definition of basal rate of metabolism. Physiol Zool 70:718–720
McNab BK (2009) Ecological factors affect the level and scaling of avian BMR. Comp Biochem Physiol A 152:22–45
McNab BK (2015) Behavioral and ecological factors account for variation in the mass-independent energy expenditures of endotherms. J Comp Physiol B 185:1–13
Møller AP (2009) Basal metabolic rate and risk-taking behaviour in birds. J Evol Biol 22:2420–2429
Montiglio P-O, Dammahn M, Messier G, Réale D (2018) The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum. Behav Ecol Sociobiol. (in press)
Nespolo RF, Bacigalupe LD, Sabat P, Bozinovic F (2002) Interplay among energy metabolism, organ mass and digestive enzyme activity in the mouse-opossum Thylamys elegans: the role of thermal acclimation. J Exp Biol 205:2697–2703
Niemelä PT, Vainikka A, Hedrick AV, Kortet R (2012) Integrating behaviour with life history: boldness of the field cricket, Gryllus integer, during ontogeny. Funct Ecol 26:450–456
Niemelä PT, Dingemanse NJ, Alioravainen N, Vainikka A, Kortet R (2013) Personality pace-of-life hypothesis: testing genetic associations among personality and life history. Behav Ecol 24:935–941
Nilsson JA (2002) Metabolic consequences of hard work. Proc R Soc Lond B 269:1735–1739
Oli MK (2004) The fast-slow continuum and mammalian life-history patterns: an empirical evaluation. Basic Appl Ecol 5:449–463
Ots I, Kerimov AB, Ivankina EV, Ilyina TA, Hõrak P (2001) Immune challenge affects basal metabolic activity in wintering great tits. Proc R Soc Lond B 268:1175–1181
Pap PL, Vágási C, Vincze O, Osváth G, Veres-Szászka J, Czirják G (2015) Physiological pace of life: the link between constitutive immunity, developmental period, and metabolic rate in European birds. Oecologia 177:147–158
Rádai Z, Kiss B, Barta Z (2017) Pace of life and behaviour: rapid development is linked with increased activity and voracity in the wolf spider Pardosa agrestis. Anim Behav 126:145–151
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos T Roy Soc B 365:4051–4063
Richter-Boix A, Llorente GA, Montori A, Garcia J (2007) Tadpole diet selection varies with the ecological context in predictable ways. Basic Appl Ecol 8:464–474
Ricklefs RE, Konarzewski M, Daan S (1996) The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am Nat 147:1047–1071
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Royauté R, Berdal M, Hickey C, Dochtermann NA (2018) Paceless life? A meta-analysis of the pace-of-life syndrome hypothesis. Behav Ecol. https://doi.org/10.1007/s00265-018-2472-z
Royauté R, Greenlee K, Baldwin M, Dochtermann NA (2015) Behaviour, metabolism and size: phenotypic modularity or integration in Acheta domesticus? Anim Behav 110:163–169
Sæther BE (1987) The influence of body weight on the covariation between reproductive traits in European birds. Oikos 48:79–88
Sæther BE, Bakke O (2000) Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81:642–653
Salguero-Gómez R, Jones OR, Jongejans E, Blomberg SP, Hodgson DJ, Mbeau-Ache C, Zuidema PA, de Kroon H, Buckley YM (2016) Fast-slow continuum and reproductive strategies structure plant life-history variation worldwide. P Natl Acad Sci USA 113:230–235
Selman C, Lumsden S, Bünger L, Hill WG, Speakman JR (2001) Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J Exp Biol 204:777–784
Shearer TA, Pruitt JN (2014) Individual differences in boldness positively correlate with heart rate in orb-weaving spiders of genus Larinioides. Curr Zool 60:387–391
Sih A (1987) Predator and prey lifestyles: an evolutionary and ecological overview. In: Predation: direct and indirect impacts on aquatic communities. (Ed. by WC Kerfoot & A Sih), pp 203–224. Hanover, New Hampshire: University Press of New England.
Skelly DK (1994) Activity level and the susceptibility of anuran larvae to predation. Anim Behav 47:465–468
Song ZG, Wang DH (2006) Basal metabolic rate and organ size in Brandt’s voles (Lasiopodomys brandtii): effects of photoperiod, temperature and diet quality. Physiol Behav 89:704–710
Speakman JR, Krol E, Johnson MS (2004) The functional significance of individual variation in basal metabolic rate. Physiol Biochem Zool 77:900–915
Stearns SC (1989) Trade-offs in life-history evolution. Funct Ecol 3:259–268
Tieleman BI, Williams JB, Ricklefs RE, Klasing KC (2005) Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc R Soc Lond B 272:1715–1720
Turbill C, Ruf T, Rothmann A, Arnold W (2013) Social dominance is associated with individual differences in heart rate and energetic response to food restriction in female red deer. Physiol Biochem Zool 86:528–537
van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life-history tactics. Am Nat 128:137–142
Vehanen T (2003) Adaptive flexibility in the behaviour of juvenile Atlantic salmon: short-term responses to food availability and threat from predation. J Fish Biol 63:1034–1045
Versteegh MA, Schwabl I, Jaquier S, Tieleman BI (2012) Do immunological, endocrine and metabolic traits fall on a single pace-of-life axis? Covariation and constraints among physiological systems. J Evol Biol 25:1864–1876
Villagra CA, Ramı́rez CC, Niemeyer HM (2002) Antipredator responses of aphids to parasitoids change as a function of aphid physiological state. Anim Behav 64:677–683
Wengström N, Wahlqvist F, Näslund J, Aldvén D, Závorka L, Osterling ME, Höjesjö J (2016) Do individual activity patterns of brown trout (Salmo trutta) alter the exposure to parasitic freshwater pearl mussel (Margaritifera margaritifera) larvae? Ethology 122:769–778
Werner EE, Anholt BR (1993) Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am Nat 142:242–272
White CR, Kearney MR (2013) Determinants of inter-specific variation in basal metabolic rate. J Comp Physiol B 183:1–26
White SJ, Kells TJ, Wilson AJ (2016) Metabolism, personality and pace of life in the Trinidadian guppy, Poecilia reticulata. Behaviour 153:1517–1543
Wolf M, McNamara JM (2012) On the evolution of personalities via frequency-dependent selection. Am Nat 179:679–692
Zanette L, Smith JNM, van Oort H, Clinchy M (2003) Synergistic effects of food and predators on annual reproductive success in song sparrows. Proc R Soc Lond B 270:799–803
Závorka L, Aldvén D, Näslund J, Höjesjö J, Johnsson JI (2015) Linking lab activity with growth and movement in the wild: explaining pace-of-life in a trout stream. Behav Ecol 26:877–884
Acknowledgments
We thank the Westneat and Crowley labs for comments throughout the process and R. Fox, J. Wright, two anonymous reviewers, and a guest editor for suggestions on the manuscript. This project emerged from a class exercise in a graduate course taught by PHC.
Funding
We received support from the Department of Biology at the University of Kentucky, and DFW received additional support from the US National Science Foundation (IOS1257718).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research did not involve either humans or animals.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by P. T. Niemelä
This article is a contribution to the Topical Collection Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life-history – Guest Editors: Melanie Dammhahn, Niels J. Dingemanse, Petri T. Niemelä, Denis Réale
Rights and permissions
About this article
Cite this article
Salzman, T.C., McLaughlin, A.L., Westneat, D.F. et al. Energetic trade-offs and feedbacks between behavior and metabolism influence correlations between pace-of-life attributes. Behav Ecol Sociobiol 72, 54 (2018). https://doi.org/10.1007/s00265-018-2460-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2460-3