Abstract
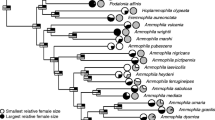
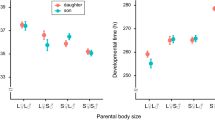
Optimal parental investment usually differs depending on the sex of the offspring. However, parents in most organisms cannot discriminate the sex of their young until those young are energetically independent. In a species with physical male–male competition, males are often larger and usually develop sexual ornaments, so male offspring are often more costly to produce. However, Onthophagus dung beetles (Coleoptera; Scarabaeidae) are highly dimorphic in secondary sexual characters, but sexually monomorphic in body size, despite strong male–male competition for mates. We demonstrate that because parents provide all resources required by their offspring before adulthood, O. atripennis exhibits no sexual size dimorphism irrespective of sexual selection pressure favoring sexual dimorphism. By constructing a graphic model with three fitness curves (for sons, daughters, and expected fitness return for parents), we demonstrate that natural selection favors parents that provide both sons and daughters with the optimal amount of investment for sons, which is far greater than that for daughters. This is because the cost of producing small sons, that are unable to compete for mates, is far greater than the cost of producing daughters that are larger than necessary. This theoretical prediction can explain sexual dimorphism without sexual size dimorphism, widely observed in species with crucial parental care such as dung beetles and leaf-rolling beetles, and may provide an insight into the enigmatic relationship between sexual size dimorphism and sexual dimorphism.






Similar content being viewed by others
References
Blanckenhorn WU (2005) Behavioral causes and consequences of sexual size dimorphism. Ethology 111:977–1016
Brandmayr P (1992) Short review of the presocial evolution in Coleoptera. Ethol Ecol Evol Special Issue 2:7–16
Carriere Y, Roff DA (1995) The evolution of offspring size and number: a test of the Smith–Fretwell model in three species of crickets. Oecologia 102:389–396
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theor Popul Biol 9:129–136
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton, New Jersey
Clutton-Brock TM (1991) The evolution of parental care. Princeton University Press, Princeton, New Jersey
Clutton-Brock TM, Albon SD, Guinness FE (1981) Parental investment in male and female offspring in polygynous mammals. Nature 289:487–489
Clutton-Brock TH, Albon SD, Guiness FE (1984) Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308:358–360
Doube BM, Giller PS, Moola F (1988) Dung burial strategies in some South African coprine and onitine dung beetles (Scarabaeidae, Scarabaeinae). Ecol Entomol 13:251–261
Eberhard WG (1980) Horned beetles. Sci Am 242:124–131
Emlen DJ (1997) Alternative reproductive tactics and male-dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav Ecol Sociobiol 41:335–341
Emlen DJ, Nijhout HF (2000) The development and evolution of exaggerated morphologies in insects. Annu Rev Entomol 45:661–708
Emlen DJ, Marangelo J, Ball B, Cunningham CW (2005a) Diversity in the weapons of sexual selection: horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae). Evolution 59:1060–1084
Emlen DJ, Hunt J, Simmons LW (2005b) Evolution of sexual dimorphism and male dimorphism in the expression of beetle horns: phylogenetic evidence for modularity, evolutionary lability, and constraint. Am Nat 166:S42–S68
Hosogi Y (1985) Ecological studies on the utilization of main coprophagous beetles in pastures in the warm area of Japan. Bulletin of the Kochi Prefectural Livestock Experiment Station 14 (in Japanese)
Hunt J, Simmons LW (1997) Patterns of fluctuating asymmetry in beetle horns: an experimental examination of the honest signalling hypothesis. Behav Ecol Sociobiol 41:109–114
Hunt J, Simmons LW (2000) Maternal and paternal effects on offspring phenotype in the dung beetle Onthophagus taurus. Evolution 54:936–941
Hunt J, Simmons LW (2004) Optimal maternal investment in the dung beetle Onthophagus taurus? Behav Ecol Sociobiol 55:302–312
Karino K, Seki N, Chiba M (2004) Larval nutritional environment determines adult size in Japanese horned beetles Allomyrina dichotoma. Ecol Res 19:663–668
Kawai S, Hori S, Kawahara M, Inagaki M (2005) Atlas of Japanese Scarabaeoidea, vol 1 Coprophagous Group. Roppon-Ashi Entomological Books, Tokyo, Japan (in Japanese)
Kawano K (2006) Sexual dimorphism and the making of oversized male characters in beetles (Coleoptera). Ann Entomol Soc Am 99:327–341
Kishi S, Nishida T (2006) Adjustment of parental investment in the dung beetle Onthophagus atripennis (Coleoptera: Scarabaeidae). Ethology 112:1239–1245
Kotiaho JS, Simmons LW, Hunt J, Tomkins JI (2003) Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am Nat 161:852–859
Koyama N, Takahata Y, Huffman MA (1992) Reproductive parameters of female Japanese macaques: thirty years of data from the Arashiyama troops, Japan. Primates 33:33–47
Kurihara T, Kurihara T (2005) Male-male combat in Onthophagus atripennis. Saikaku Tsushin 10:21–28 (in Japanese)
Lee JM, Peng Y (1982) Influence of manure availability and nesting density on the progeny size of Onthophagus gazella. Environ Entomol 11:38–41
Moczek AP (1998) Horn polyphenism in the beetle Onthophagus taurus: larval diet quality and plasticity in parental investment determine adult body size and male horn morphology. Behav Ecol 9:636–641
Moczek AP, Emlen DJ (1999) Proximate determination of male horn dimorphism in the beetle Onthophagus taurus (Coleoptera: Scarabaeidae). J Evol Biol 12:27–37
Palestrini C, Roland A (2001) Body size and paternal investment in the genus Onthophagus (Coleoptera,Scarabaeoidea). J Zool 255:405–412
Palmer TJ (1978) A horned beetle which fights. Nature 274:583–584
Paredes R, Jones IL, Boness DJ (2005) Reduced parental care, compensatory behaviour and reproductive costs of thick-billed murres equipped with data loggers. Anim Behav 69:197–208
Parker GA, Stuart RA (1976) Animal behavior as a strategy optimizer: evolution of resource assessment strategies and optimal emigration thresholds. Am Nat 110:1055–1076
Sakurai K (1990) Leaf size recognition and evaluation by some attelabid weevils (3) Deporaus sp. Behaviour 115:348–370
Sargent RC, Taylor PD, Gross MR (1987) Parental care and the evolution of egg size in fishes. Am Nat 129:32–46
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Trumbo ST, Sikes DS (2000) Sexual selection and leg morphology in Nicrophorus orbicillis and Ptomascopus morio. Entomol Sci 3:585–589
Vitturi R, Colomba M, Volpe N, Lannino A, Zunino M (2003) Evidence for male XO sex-chromosome system in Pentodon bidens punctatum (Coleoptera Scarabaeoidea: Scarabaeidae) with X-linked 18S-28S rDNA clusters. Genes Genet Syst 78:427–432
Acknowledgments
We thank the staff of the Arashiyama Monkey Park and the Nara Park Management Office for permission to collect monkey and deer dung, respectively, and dung beetles. For valuable advice and discussions, we thank K. Fujisaki, A. Kochi and other fellows in our laboratory. K. Takakura for invaluable comments and corrections, two anonymous reviewers for helpful comments on previous versions of the manuscript. This work was supported in part by the 21st Century COE program for Innovative Food and Environmental Studies Pioneered by Entomomimetic Sciences, of the Ministry of Education, Culture, Sports, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: N. Wedell
Rights and permissions
About this article
Cite this article
Kishi, S., Nishida, T. Optimal investment in sons and daughters when parents do not know the sex of their offspring. Behav Ecol Sociobiol 62, 607–615 (2008). https://doi.org/10.1007/s00265-007-0485-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-007-0485-0