Abstract
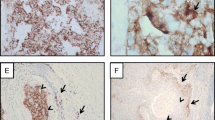
The dynamics of PD-L1 expression are poorly understood over the development of lung adenocarcinomas from pre-invasive lesions to fully invasive carcinomas. Given the importance of PD-L1 expression for the selection of patients to receive immunotherapy in the metastatic setting and possibly in the neoadjuvant setting, we sought to evaluate the agreement of PD-L1 expression in invasive and lepidic components of resected tumor specimens. We stained 86 adenocarcinomas for PD-L1 using the SP263 clone. We assessed the agreement of PD-L1 expression by tumor cells and immune cells between lepidic and invasive components. When both lepidic and invasive components were considered, PD-L1 positive immune cells and tumor cells were observed in 50 (58.1%) and 18 (20.9%) samples, respectively, using a ≥ 1% PD-L1 expression cutoff. Using a ≥ 1% cutoff for PD-L1 expression, positively stained tumor cells were observed in 11 (13%) lepidic and 15 (17%) invasive patterns, with agreement in 76 (88%) specimens and disagreement in 10 (12%) specimens (ĸ = 0.549). At ≥ 1% PD-L1 expression cutoff, PD-L1 positive immune cells were observed in 31 (35%) lepidic and 32 (37%) invasive patterns with an agreement of PD-L1 expression in 49 (57%) specimens and disagreement in 37 (43%) specimens (ĸ = 0.073). In our study of early stage adenocarcinomas of the lung, there was poor agreement in PD-L1 expression between paired invasive and lepidic components of tumors. Our data suggest that the non-invasive tumor components may not be as immunostimulatory as the invasive components, resulting in less adaptive expression of PD-L1.



Similar content being viewed by others
References
Langer CJ et al (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17(11):1497–1508
Paz-Ares L et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051
Reck M et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833
Antonia SJ et al (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379(24):2342–2350
Kim MY et al (2015) Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 88(1):24–33
Kluger HM et al (2015) Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res 21(13):3052–3060
Madore J et al (2015) PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 28(3):245–253
Lee SM et al (2013) Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology 268(1):265–273
Jones KD (2013) Whence lepidic?: the history of a Canadian neologism. Arch Pathol Lab Med 137(12):1822–1824
Travis WD et al (2015) The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10(9):1243–1260
Araki K et al (2014) Excellent prognosis of lepidic-predominant lung adenocarcinoma: low incidence of lymphatic vessel invasion as a key factor. Anticancer Res 34(6):3153–3156
Xu L, Tavora F, Burke A (2013) Histologic features associated with metastatic potential in invasive adenocarcinomas of the lung. Am J Surg Pathol 37(7):1100–1108
Yanagawa N et al (2013) New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 8(5):612–618
Blomberg OS, Spagnuolo L, de Visser KE (2018) Immune regulation of metastasis: mechanistic insights and therapeutic opportunities. Dis Model Mech 11(10):dmm036236. https://doi.org/10.1242/dmm.036236
Mansfield AS et al (2016) Heterogeneity of programmed cell death ligand 1 expression in multifocal lung cancer. Clin Cancer Res 22(9):2177–2182
Hirsch FR et al (2017) PD-L1 Immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12(2):208–222
Hellmann MD et al (2017) Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 18(1):31–41
Krzywinski M et al (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19(9):1639–1645
Ng Kee Kwong F et al (2018) Expression of PD-L1 correlates with pleomorphic morphology and histological patterns of non-small-cell lung carcinomas. Histopathology. 72(6):1024–1032
Terra S et al (2017) Temporal and spatial heterogeneity of programmed cell death 1-Ligand 1 expression in malignant mesothelioma. Oncoimmunology 6(11):e1356146
Terra S et al (2019) Heterogeneity of programmed death-ligand 1 expression in thymic epithelial tumours between initial specimen and synchronous or metachronous metastases or recurrences. Histopathology 74(2):364–367
Ilie M et al (2016) Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 27(1):147–153
Mansfield AS et al (2016) Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol 27(10):1953–1958
Miyazawa T et al (2019) PD-L1 expression in non-small-cell lung cancer including various adenocarcinoma subtypes. Ann Thorac Cardiovasc Surg 25(1):1–9
Naso JR et al (2020) Intratumoral heterogeneity in programmed death-ligand 1 immunoreactivity is associated with variation in non-small cell lung carcinoma histotype. Histopathology 76(3):394–403
Jiang X et al (2019) Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 18(1):10
Mansfield AS et al (2018) Contraction of T cell richness in lung cancer brain metastases. Sci Rep 8(1):2171
Yu H et al (2016) PD-L1 expression in lung cancer. J Thorac Oncol 11(7):964–975
Adam J et al (2018) Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol 29(4):953–958
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hazim, A., Majithia, N., Murphy, S.J. et al. Heterogeneity of PD-L1 expression between invasive and lepidic components of lung adenocarcinomas. Cancer Immunol Immunother 70, 2651–2656 (2021). https://doi.org/10.1007/s00262-021-02883-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02883-x