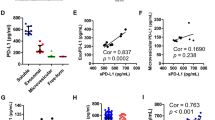
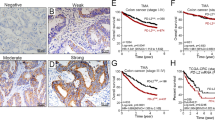
Abstract
Background
Programmed cell death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) play a pivotal role in cancer immunotherapy. Each of these molecules has a membrane-bound receptor form (mPD-L1/mCTLA-4) and a soluble form (sPD-L1/sCTLA-4). However, these prognostic impacts in colorectal cancer (CRC) remain unclear.
Methods
We immunohistochemically scored tumoral mPD-L1/mCTLA-4 expression and quantified preoperative circulating sPD-L1/sCTLA-4 levels using matched serum specimens from 131 patients with pStage I–III CRC. We also examined the association between these statuses and tumor infiltrating lymphocytes (TILs) in these patients.
Results
Elevated levels of mPD-L1, mCTLA-4, sPD-L1 and sCTLA-4 were significantly correlated with poor overall survival (OS) and disease-free survival (DFS). Co-high expression of tumoral mPD-L1 and mCTLA-4 or co-elevated levels of serum sPD-L1 and sCTLA-4 were strongly correlated with poor OS and DFS. Multivariate analysis revealed that both statuses were negative independent prognostic factors for OS [hazard ratio (HR) 3.86, 95% confidence interval (95% CI) 1.71–8.51, p = 0.001; HR 5.72, 95% CI 1.87–14.54, p = 0.004, respectively] and DFS (HR 2.53, 95% CI 1.23–4.95, p = 0.01; HR 6.88, 95% CI 2.42–17.13, p = 0.0008, respectively). Although low expression of tumoral mCTLA-4 was significantly correlated with increased CD8(+) TILs, there was no correlation in any other combination.
Conclusions
We verified the prognostic impacts of mPD-L1, mCTLA-4, sPD-L1 and sCTLA-4 in pStage I–III CRC patients. Dual evaluation of immune checkpoint molecules in primary tissues or preoperative serum could identify a patient population with poor prognosis in these patients.



Similar content being viewed by others
Abbreviations
- CRC:
-
Colorectal cancer
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated antigen 4
- DFS:
-
Disease-free survival
- FFPE:
-
Formalin-fixed, paraffin-embedded
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratios
- mPD-L1:
-
Membrane-bound PD-L1
- mCTLA-4:
-
Membrane-bound CTLA-4
- MSI:
-
Microsatellite unstable
- MSS:
-
Microsatellite Stable
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death ligand-1
- ROC:
-
Receiver operating characteristic
- sPD-L1:
-
Soluble PD-L1
- sCTLA-4:
-
Soluble CTLA-4
- TME:
-
Tumor microenvironment
- Treg cells:
-
Regulatory T cells
- TILs:
-
Tumor infiltrating lymphocytes
- TNM:
-
Tumor–Node–Metastasis
- 95% CI:
-
95% confidence interval
References
Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64:104–117. https://doi.org/10.3322/caac.21220
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520. https://doi.org/10.1056/NEJMoa1500596
Couzin-Frankel J (2013) Breakthrough of the year 2013. Cancer Immunother Sci 342:1432–1433. https://doi.org/10.1126/science.342.6165.1432
Goto M, Chamoto K, Higuchi K et al (2019) Analytical performance of a new automated chemiluminescent magnetic immunoassays for soluble PD-1, PD-L1, and CTLA-4 in human plasma. Sci Rep 9:10144. https://doi.org/10.1038/s41598-019-46548-3
Curran MA, Montalvo W, Yagita H, Allison JP (2010) PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 107:4275–4280. https://doi.org/10.1073/pnas.0915174107
Huang PY, Guo SS, Zhang Y et al (2016) Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget 7:13060–13068. https://doi.org/10.18632/oncotarget.7421
Zhang XF, Pan K, Weng DS et al (2016) Cytotoxic T lymphocyte antigen-4 expression in esophageal carcinoma: implications for prognosis. Oncotarget 7:26670–26679. https://doi.org/10.18632/oncotarget.8476
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. https://doi.org/10.1056/NEJMoa1003466
Antonia S, Goldberg SB, Balmanoukian A et al (2016) Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 17:299–308. https://doi.org/10.1016/s1470-2045(15)00544-6
O’Reilly EM, Oh DY, Dhani N et al (2019) Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1588
Chang B, Huang T, Wei H et al (2019) The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother CII 68:353–363. https://doi.org/10.1007/s00262-018-2271-4
Li Y, Xiao Y, Su M, Zhang R, Ding J, Hao X, Ma Y (2016) Role of soluble programmed death-1 (sPD-1) and sPD-ligand 1 in patients with cystic echinococcosis. Exp Ther Med 11:251–256. https://doi.org/10.3892/etm.2015.2876
Pistillo MP, Fontana V, Morabito A et al (2019) Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: an Italian melanoma intergroup study. Cancer Immunol Immunother CII 68:97–107. https://doi.org/10.1007/s00262-018-2258-1
Shigemori T, Toiyama Y, Okugawa Y et al (2019) Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol 26:876–883. https://doi.org/10.1245/s10434-018-07112-x
Zhang M, Li G, Wang Y, Wang Y, Zhao S, Haihong P, Zhao H, Wang Y (2017) PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep 7:10255. https://doi.org/10.1038/s41598-017-10925-7
Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, Zhang Q (2017) Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 8:31347–31354. https://doi.org/10.18632/oncotarget.15532
Zhang P, Ouyang S, Wang J, Huang Z, Wang J, Liao L (2015) Levels of programmed death-1 and programmed death ligand-1 in the peripheral blood of patients with oral squamous cell carcinoma and its clinical implications. Hua Xi Kou Qiang Yi Xue Za Zhi 33:529–533
Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58:3491–3494
Martin F, Ladoire S, Mignot G, Apetoh L, Ghiringhelli F (2010) Human FOXP3 and cancer. Oncogene 29:4121–4129. https://doi.org/10.1038/onc.2010.174
Saito T, Nishikawa H, Wada H et al (2016) Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 22:679–684. https://doi.org/10.1038/nm.4086
Shang B, Liu Y, Jiang S-j, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5:15179. https://doi.org/10.1038/srep15179
Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C (2020) The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: a systematic review and meta-analysis. Sci Rep 10:3360. https://doi.org/10.1038/s41598-020-60255-4
Toiyama Y, Tanaka K, Kitajima T et al (2014) Elevated serum angiopoietin-like protein 2 correlates with the metastatic properties of colorectal cancer: a serum biomarker for early diagnosis and recurrence. Clin Cancer Res 20:6175–6186. https://doi.org/10.1158/1078-0432.ccr-14-0007
Goel A, Nagasaka T, Hamelin R, Boland CR (2010) An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS One 5:e9393. https://doi.org/10.1371/journal.pone.0009393
Kim JW, Nam KH, Ahn SH et al (2016) Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 19:42–52. https://doi.org/10.1007/s10120-014-0440-5
Ward FJ, Dahal LN, Wijesekera SK, Abdul-Jawad SK, Kaewarpai T, Xu H, Vickers MA, Barker RN (2013) The soluble isoform of CTLA-4 as a regulator of T-cell responses. Eur J Immunol 43:1274–1285. https://doi.org/10.1002/eji.201242529
Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, Li Q, Cai S (2016) Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer 15:55. https://doi.org/10.1186/s12943-016-0539-x
Masugi Y, Nishihara R, Yang J et al (2017) Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut 66:1463–1473. https://doi.org/10.1136/gutjnl-2016-311421
Contardi E, Palmisano GL, Tazzari PL et al (2005) CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer 117:538–550. https://doi.org/10.1002/ijc.21155
Chen X, Shao Q, Hao S, Zhao Z, Wang Y, Guo X, He Y, Gao W, Mao H (2017) CTLA-4 positive breast cancer cells suppress dendritic cells maturation and function. Oncotarget 8:13703–13715. https://doi.org/10.18632/oncotarget.14626
Chau GY, Wu CW, Lui WY et al (2000) Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg 231:552–558. https://doi.org/10.1097/00000658-200004000-00015
Gu D, Ao X, Yang Y, Chen Z, Xu X (2018) Soluble immune checkpoints in cancer: production, function and biological significance. J Immunother Cancer 6:132. https://doi.org/10.1186/s40425-018-0449-0
Ward FJ, Dahal LN, Khanolkar RC, Shankar SP, Barker RN (2014) Targeting the alternatively spliced soluble isoform of CTLA-4: prospects for immunotherapy? Immunotherapy 6:1073–1084. https://doi.org/10.2217/imt.14.73
Kushlinskii NE, Gershtein ES, Morozov AA, Goryacheva IO, Filipenko ML, Alferov AA, Bezhanova SD, Bazaev VV, Kazantseva IA (2019) Soluble ligand of the immune checkpoint receptor (sPD-L1) in blood serum of patients with renal cell carcinoma. Bull Exp Biol Med 166:353–357. https://doi.org/10.1007/s10517-019-04349-8
Yang J, Hu M, Bai X, Ding X, Xie L, Ma J, Fan B, Yu J (2019) Plasma levels of soluble programmed death ligand 1 (sPD-L1) in WHO II/III nasopharyngeal carcinoma (NPC): a preliminary study. Medicine (Baltimore) 98:e17231. https://doi.org/10.1097/md.0000000000017231
Zheng Z, Bu Z, Liu X et al (2014) Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res 26:104–111. https://doi.org/10.3978/j.issn.1000-9604.2014.02.08
Ando K, Hamada K, Watanabe M et al (2019) Plasma levels of soluble PD-L1 correlate with tumor regression in patients with lung and gastric cancer treated with immune checkpoint inhibitors. Anticancer Res 39:5195–5201. https://doi.org/10.21873/anticanres.13716
Dienstmann R, Mason MJ, Sinicrope FA et al (2017) Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol 28:1023–1031. https://doi.org/10.1093/annonc/mdx052
Taieb J, Le Malicot K, Shi Q et al (2017) Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djw272
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765. https://doi.org/10.1056/NEJMoa0804385
Wu M, Kim YS, Ryu H-S et al (2020) MSI status is associated with distinct clinicopathological features in BRAF mutation colorectal cancer: a systematic review and meta-analysis. Pathol Res Pract 216:152791. https://doi.org/10.1016/j.prp.2019.152791
Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M (2016) PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol 29:1104–1112. https://doi.org/10.1038/modpathol.2016.95
Shin SJ, Kim SY, Choi YY, Son T, Cheong JH, Hyung WJ, Noh SH, Park CG, Kim HI (2019) Mismatch repair status of gastric cancer and its association with the local and systemic immune response. Oncologist 24:e835–e844. https://doi.org/10.1634/theoncologist.2018-0273
Fiegle E, Doleschel D, Koletnik S, Rix A, Weiskirchen R, Borkham-Kamphorst E, Kiessling F, Lederle W (2019) Dual CTLA-4 and PD-L1 blockade inhibits tumor growth and liver metastasis in a highly aggressive orthotopic mouse model of colon cancer. Neoplasia 21:932–944. https://doi.org/10.1016/j.neo.2019.07.006
Overman MJ, Lonardi S, Wong KYM et al (2019) Nivolumab (NIVO) + low-dose ipilimumab (IPI) in previously treated patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): long-term follow-up. J Clin Oncol 37:635. https://doi.org/10.1200/jco.2019.37.4_suppl.635
Acknowledgements
We thank all the patients, their families, and the investigators involved in this study. We also thank Mrs. Yuki Orito and Mrs. Amphone Okada for their excellent technical assistance and R J Frampton from Edanz Group for editing a draft of this manuscript.
Funding
The work was partially supported by a Grant in Aid for Scientific Research (16K10533, 18K08592) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Study conception and design: YuO, YT, YOO, and MK; acquisition and analysis of the data: YuO, YT, YoO, AY, CY, KK, YK, TS, SI, TK, HF, HY, JH, and MO; interpretation of the data: YUO, YT, and YOO; drafting of the manuscript: YUO, YT, YOO, and MK; critical revision of the manuscript: YUO, YT, YOO, AY, CY, KK, YK, TS, SI, TK, HF, HY, JH, MO, and MK.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Consent to participate
Written informed consent was obtained from all patients in accordance with guidelines approved by the Institutional Review Board of Mie University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure
1. Representative images of tumoral mPD-L1 and mCTLA-4 expression in CRC cells. (a–d) PD-L1; (a) absent (b) weak (c) moderate (d) strong, 100 × magnification (e–h) CTLA-4; (e) absent (f) weak (g) moderate (h) strong, 100 × magnification (PDF 490 kb)
Supplementary Figure
2. Representative images of PD-L1, CTLA-4, CD8 and FoxP3 (+) T cells with Cellsens software imaging system in CRC tissue. (a) PD-L1(+) T cells (b) CTLA-4(+) T cells, (c) CD8(+) T cells (d) FoxP3(+) T cells 100 × magnification (PDF 293 kb)
Supplementary Figure
3. Receiver operating characteristic (ROC) curve analysis for overall survival to decide the cut-off value of tumoral and circulating expression of immune checkpoints. (a) mPD-L1 (sensitivity: 0.92, specificity: 0.28, AUC: 0.57, cut-off: 4 (score of expression)) (b) mCTLA-4 (sensitivity: 0.48, specificity: 0.86, AUC: 0.65, cut-off: 6 (score of expression)) (c) sPD-L1 (sensitivity: 0.40, specificity: 0.86, AUC: 0.59, cut-off: 0.08 (ng/ml)) (d) sCTLA-4 (sensitivity: 0.32, specificity: 0.87, AUC: 0.51, cut-off: 1.79 (ng/ml)) (PDF 144 kb)
Rights and permissions
About this article
Cite this article
Omura, Y., Toiyama, Y., Okugawa, Y. et al. Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patients. Cancer Immunol Immunother 69, 2533–2546 (2020). https://doi.org/10.1007/s00262-020-02645-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02645-1