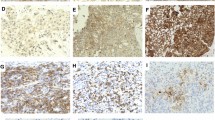
Abstract
Background
Melanoma-associated antigen-A (MAGE-A) and programmed-death ligand 1 (PD-L1) are present in urothelial carcinoma (UC). We assessed survival outcomes in patients with MAGE-A and PD-L1 expression.
Methods
MAGE-A and PD-L1 expression on neoplastic cells was analyzed using tissue microarrays from patients with UC. We compared differential expression between disease stage and grade. MAGE-A and PD-L1 co-expression was subcategorized. Fisher’s exact test was done for categorical variables followed by univariable and multivariable analysis of recurrence-free survival (RFS) and progression-free survival (PFS).
Results
Co-expression of MAGE+/PD-L1+ was higher in advanced disease; however, only MAGE+/PD-L1− was associated with shorter RFS [hazard ratio (HR) 1.89; 95% confidence interval (CI) 1.19–2.99; p = .006]. MAGE+/PD-L1+ was associated with the worst PFS (HR 17.1; 95% CI 5.96–49.4; p ≤ .001). MAGE-A expression was more prevalent with high-grade (p = .015), and higher-stage ≥ pT2 (p = .001) disease. The 5-year RFS was 44% for MAGE+ versus 58% for MAGE− patients. On multivariable analysis, MAGE+ was also associated with shorter RFS (HR 1.55; 95% CI 1.05–2.30; p = .03). Similarly, MAGE+ was associated with shorter PFS (HR 3.12; 95% CI 1.12–8.68; p = .03).
Conclusion
MAGE-A and PD-L1 expression is increased in advanced disease and associated with shorter PFS. Furthermore, MAGE-A expression was significantly associated with higher-grade and -stage disease and associated with shorter RFS and PFS. The worse prognosis associated with MAGE-A+/PD-L1+ provides evidence that a combinatorial treatment strategy co-targeting MAGE/PD-L1 might be feasible. Further studies are needed to validate these findings.


Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- IQR:
-
Inter-quartile range
- MAGE-A:
-
Melanoma-associated antigen-A
- MP:
-
MAGE-positive
- MN:
-
MAGE-negative
- PD-L1:
-
Programmed death-ligand 1
- RC:
-
Radical cystectomy
- RFS:
-
Recurrence-free survival
- TCR:
-
Adoptive T-cell receptor-engineered T-cell therapy
- TMA:
-
Tissue microarray
- UC:
-
Urothelial carcinoma
References
Sang M, Wang L, Ding C, Zhou X, Wang B, Wang L, Lian Y, Shan B (2011) Melanoma-associated antigen genes—an update. Cancer Lett 302:85–90. https://doi.org/10.1016/j.canlet.2010.10.021
Yin B, Zeng Y, Liu G, Wang X, Wang P, Song Y (2014) MAGE-A3 is highly expressed in a cancer stem cell-like side population of bladder cancer cells. Int J Clin Exp Pathol 7:2934–2941
Yin B, Liu G, Wang XS, Zhang H, Song YS, Wu B (2012) Expression profile of cancer-testis genes in transitional cell carcinoma of the bladder. Urol Oncol 30:886–892. https://doi.org/10.1016/j.urolonc.2010.08.017
Dyrskjot L, Zieger K, Kissow Lildal T, Reinert T, Gruselle O, Coche T, Borre M, Orntoft TF (2012) Expression of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br J Cancer 107:116–122. https://doi.org/10.1038/bjc.2012.215
Kerkar SP, Wang ZF, Lasota J, Park T, Patel K, Groh E, Rosenberg SA, Miettinen MM (2016) MAGE-A is more highly expressed than NY-ESO-1 in a systematic immunohistochemical analysis of 3668 cases. J Immunother 39:181–187. https://doi.org/10.1097/CJI.0000000000000119
Xylinas E, Cha EK, Khani F, Kluth LA, Rieken M, Volkmer BG, Hautmann R, Küfer R, Chen YT, Zerbib M, Rubin MA, Scherr DS, Shariat SF, Robinson BD (2014) Association of oncofetal protein expression with clinical outcomes in patients with urothelial carcinoma of the bladder. J Urol 191:830–841. https://doi.org/10.1016/j.juro.2013.08.048
Sharma P, Shen Y, Wen S, Bajorin DF, Reuter VE, Old LJ, Jungbluth AA (2006) Cancer-testis antigens: expression and correlation with survival in human urothelial carcinoma. Clin Cancer Res 12:5442–5447. https://doi.org/10.1158/1078-0432.CCR-06-0527
Lu YC, Parker LL, Lu T, Zheng Z, Toomey MA, White DE, Yao X, Li YF, Robbins PF, Feldman SA, van der Bruggen P, Klebanoff CA, Goff SL, Sherry RM, Kammula US, Yang JC, Rosenberg SA (2017) Treatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol 35:3322–3329. https://doi.org/10.1200/JCO.2017.74.5463
Faiena I, Cummings AL, Crosetti AM, Pantuck AJ, Chamie K, Drakaki A (2018) Durvalumab: an investigational anti-PD-L1 monoclonal antibody for the treatment of urothelial carcinoma. Drug Des Devel Ther 12:209–215. https://doi.org/10.2147/DDDT.S141491
Yoon DH, Osborn MJ, Tolar J, Kim CJ (2018) Incorporation of immune checkpoint blockade into chimeric antigen receptor T cells (CAR-Ts): combination or built-in CAR-T. Int J Mol Sci. https://doi.org/10.3390/ijms19020340
Moon EK, Ranganathan R, Eruslanov E, Kim S, Newick K, O’Brien S, Lo A, Liu X, Zhao Y, Albelda SM (2016) Blockade of programmed death 1 augments the ability of human T cells engineered to target NY-ESO-1 to control tumor growth after adoptive transfer. Clin Cancer Res 22:436–447. https://doi.org/10.1158/1078-0432.CCR-15-1070
John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH, Darcy PK (2013) Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res 19:5636–5646. https://doi.org/10.1158/1078-0432.CCR-13-0458
Mullinax JE, Hall M, Prabhakaran S, Weber J, Khushalani N, Eroglu Z, Brohl AS, Markowitz J, Royster E, Richards A, Stark V, Zager JS, Kelley L, Cox C, Sondak VK, Mulé JJ, Pilon-Thomas S, Sarnaik AA (2018) Combination of ipilimumab and adoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic melanoma. Front Oncol 8:44. https://doi.org/10.3389/fonc.2018.00044
Kodumudi KN, Siegel J, Weber AM, Scott E, Sarnaik AA, Pilon-Thomas S (2016) Immune checkpoint blockade to improve tumor infiltrating lymphocytes for adoptive cell therapy. PLoS One 11:e0153053. https://doi.org/10.1371/journal.pone.0153053
Bjoern J, Lyngaa R, Andersen R, Rosenkrantz LH, Hadrup SR, Donia M, Svane IM (2017) Influence of ipilimumab on expanded tumour derived T cells from patients with metastatic melanoma. Oncotarget 8:27062–27074. https://doi.org/10.18632/oncotarget.16003
Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
Faiena I, Kroeger N, Fussek S, Astrow S, Jain R, Bot A, Drakaki A (2017) MP48-02 melanoma-associated antigen-A and programmed death-ligand 1 expression in urothelial carcinoma. J Urol 197:e647 (abstract)
Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, Al-Masri H, Rebelatto MC, Walker J (2017) Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non-small cell lung cancer. Clin Cancer Res 23:3585–3591. https://doi.org/10.1158/1078-0432.CCR-16-2375
Harrell FE, Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387. https://doi.org/10.1002/(SICI)1097-0258(19960229)15:4%3C361::AID-SIM168%3E3.0.CO;2-4
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, van der Heijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Durán I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE, Boyd Z, Bourgon R, Hedge PS, Mariathasan S, Thåström A, Abidoye OO, Fine GD, Bajorin DF, Imvigor210 Study Group (2017) Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389:67–76. https://doi.org/10.1016/S0140-6736(16)32455-2
Park TS, Groh EM, Patel K, Kerkar SP, Lee CC, Rosenberg SA (2016) Expression of MAGE-A and NY-ESO-1 in primary and metastatic cancers. J Immunother 39:1–7. https://doi.org/10.1097/CJI.0000000000000101
Irving M, Vuillefroy de Silly R, Scholten K, Dilek N, Coukos G (2017) Engineering chimeric antigen receptor T-cells for racing in solid tumors: don’t forget the fuel. Front Immunol 8:267. https://doi.org/10.3389/fimmu.2017.00267
Funding
Research funding was provided by Kite, a Gilead Company. Biomedical editing was sponsored by Kite, a Gilead Company.
Author information
Authors and Affiliations
Contributions
IF: Conception, data collection, data analysis, drafting manuscript, critical revisions. SHA: Conception, drafting manuscript, critical revisions. DAE: Data analysis, drafting manuscript, critical revisions. RJ: Drafting manuscript, critical revisions. AB: Conception, drafting manuscript, critical revisions. KC: Conception, drafting manuscript, critical revisions. ASB MD: Conception, critical revisions. AJP: Conception, drafting manuscript, critical revisions. AD: Conception, drafting manuscript, critical revisions.
Corresponding author
Ethics declarations
Conflict of interest
Izak Faiena and Alexandra Drakaki received research funding from Kite, a Gilead Company. Stephanie H. Astrow, Rajul Jain, and Adrian Bot are employees of Kite, a Gilead Company, and have equity ownership in Gilead Sciences, Inc. Arie S. Belldegrun is the founder and was formerly Chief Executive Officer of Kite, a Gilead Company, and has equity ownership in Gilead Sciences, Inc. Allan J. Pantuck has equity ownership in Gilead Sciences, Inc. The authors declare they have no other conflicts of interest.
Ethical approval and ethical standards
This study included de-identified data in a tissue microarray and was deemed to be an exempt study by the institutional review board (IRB #99–233) of the University of California, Los Angeles for TMA construction and data analysis; therefore, special ethical permission was not required. Requirement for consent was waived given the retrospective, de-identified nature of the samples, and the impracticality of consenting for samples stored prior to 1998.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faiena, I., Astrow, S.H., Elashoff, D.A. et al. Melanoma-associated antigen-A and programmed death-ligand 1 expression are associated with advanced urothelial carcinoma. Cancer Immunol Immunother 68, 743–751 (2019). https://doi.org/10.1007/s00262-019-02316-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02316-w