Abstract
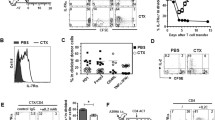
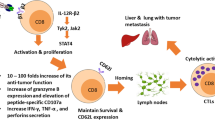
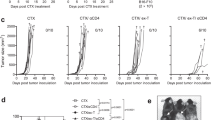
Optimal ex vivo expansion protocols for adoptive cell therapy (ACT) must yield T cells able to effectively home to tumors and survive the inhospitable conditions of the tumor microenvironment (TME), while simultaneously exerting persistent anti-tumor effector functions. Our previous work has shown that ex vivo activation in the presence of IL-12 can induce optimal expansion of murine CD8+ T cells, thus resulting in significant tumor regression after ACT mostly via sustained secretion of IFN-γ. In this report, we further elucidate the mechanism of this potency, showing that IL-12 additionally counteracts the negative regulatory effects of autocrine IFN-γ. IL-12 not only downregulates PD-1 expression by T cells, thus minimizing the effects of IFN-γ-induced PD-L1 upregulation by tumor stromal cells, but also inhibits IFNγR2 expression, thereby protecting T cells from IFN-γ-induced cell death. Thus, the enhanced anti-tumor activity of CD8+ T cells expanded ex vivo in the presence of IL-12 is due not only to the ability of IL-12-stimulated cells to secrete sustained levels of IFN-γ, but also to the additional capacity of IL-12 to counter the negative regulatory effects of autocrine IFN-γ.






Similar content being viewed by others
Abbreviations
- EthD-1:
-
Ethidium homodimer-1
- qPCR:
-
Quantitative polymerase chain reaction
References
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME (2008) Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 8(4):299–308. https://doi.org/10.1038/nrc2355
Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF (1999) Inflammatory cytokines provide a third signal for activation of naive CD4 + and CD8+ T cells. J Immunol 162(6):3256–3262
Valenzuela J, Schmidt C, Mescher M (2002) The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol 169(12):6842–6849
Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z (2006) Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 211:81–92
Chowdhury FZ, Ramos HJ, Davis LS, Forman J, Farrar JD (2011) IL-12 selectively programs effector pathways that are stably expressed in human CD8+ effector memory T cells in vivo. Blood 118(14):3890–3900. https://doi.org/10.1182/blood-2011-05-357111
Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM (2008) IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol 181(12):8576–8584
Pearce EL, Shen H (2007) Generation of CD8 T cell memory is regulated by IL-12. J Immunol 179(4):2074–2081
Starbeck-Miller GR, Xue HH, Harty JT (2014) IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med 211(1):105–120. https://doi.org/10.1084/jem.20130901
Diaz-Montero CM, Naga O, Zidan AA, Salem ML, Pallin M, Parmigiani A, Walker G, Wieder E, Komanduri K, Cole DJ, Montero AJ, Lichtenheld MG (2011) Synergy of brief activation of CD8 T-cells in the presence of IL-12 and adoptive transfer into lymphopenic hosts promotes tumor clearance and anti-tumor memory. Am J Cancer Res 1(7):882–896
Diaz-Montero CM, Zidan AA, Pallin MF, Anagnostopoulos V, Salem ML, Wieder E, Komanduri K, Montero AJ, Lichtenheld MG (2013) Understanding the biology of ex vivo-expanded CD8 T cells for adoptive cell therapy: role of CD62L. Immunol Res. https://doi.org/10.1007/s12026-013-8456-1
Diaz-Montero CM, El Naggar S, Al Khami A, El Naggar R, Montero AJ, Cole DJ, Salem ML (2008) Priming of naive CD8+ T cells in the presence of IL-12 selectively enhances the survival of CD8(+)CD62L (hi) cells and results in superior anti-tumor activity in a tolerogenic murine model. Cancer Immunol Immunother 57(4):563–572
Tewari K, Nakayama Y, Suresh M (2007) Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J Immunol 179(4):2115–2125
Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A (2017) Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep 19(6):1189–1201. https://doi.org/10.1016/j.celrep.2017.04.031
Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF (2013) Cutting edge: IL-12 and type I IFN differentially program CD8 T cells for programmed death 1 re-expression levels and tumor control. J Immunol 191(3):1011–1015. https://doi.org/10.4049/jimmunol.1300652
Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O’Donnell-Tormey J, Odunsi K, Old LJ, Ottenhoff TH, Ottensmeier C, Pawelec G, Roederer M, Roep BO, Romero P, van der Burg SH, Walter S, Hoos A, Davis MM (2012) T cell assays and MIATA: the essential minimum for maximum impact. Immunity 37(1):1–2. https://doi.org/10.1016/j.immuni.2012.07.010
Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP (1998) gp100/pmel 17 is a murine tumor rejection antigen: induction of “self’’-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med 188(2):277–286
Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M (2015) IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer 112(9):1501–1509. https://doi.org/10.1038/bjc.2015.101
Bernabei P, Coccia EM, Rigamonti L, Bosticardo M, Forni G, Pestka S, Krause CD, Battistini A, Novelli F (2001) Interferon-gamma receptor 2 expression as the deciding factor in human T, B, and myeloid cell proliferation or death. J Leukoc Biol 70(6):950–960
Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M (1993) Immune response in mice that lack the interferon-gamma receptor. Sci (N Y) 259(5102):1742–1745
Kim JW, Tsukishiro T, Johnson JT, Whiteside TL (2004) Expression of pro- and antiapoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 10(15):5101–5110. https://doi.org/10.1158/1078-0432.CCR-04-0309
Dudley ME, Rosenberg SA (2003) Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 3(9):666–675
Jiang Y, Li Y, Zhu B (2015) T-cell exhaustion in the tumor microenvironment. Cell Death Dis 6:e1792. https://doi.org/10.1038/cddis.2015.162
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499. https://doi.org/10.1038/nri3862
Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, Maini MK (2013) The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog 9(3):e1003208. https://doi.org/10.1371/journal.ppat.1003208
Refaeli Y, Van Parijs L, Alexander SI, Abbas AK (2002) Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med 196(7):999–1005
Lohman BL, Welsh RM (1998) Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J Virol 72(10):7815–7821
Novelli F, Bernabei P, Ozmen L, Rigamonti L, Allione A, Pestka S, Garotta G, Forni G (1996) Switching on of the proliferation or apoptosis of activated human T lymphocytes by IFN-gamma is correlated with the differential expression of the alpha- and beta-chains of its receptor. J Immunol 157(5):1935–1943
Funding
This work was supported by National Cancer Institute Grants K01CA134927, R21CA188767 and by a Velosano Pilot Research Award.
Author information
Authors and Affiliations
Contributions
LL performed most of the experiments, analyzed the data, and drafted the manuscript. PR and PGP Jr. assisted and/or performed some of the experiments, generated figures, and revised the manuscript. CT, TH, AM, and JF contributed to the design and interpretation of the experiments, and revised the manuscript. JK, BG, and ME oversaw the consent and collection of human samples, contributed to the design and analysis of the experiments, and revised the manuscript. CMD-M coordinated the design and execution of the experiments, data analysis, and manuscript drafting and revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animals were housed under specific pathogen-free conditions in accordance with Institutional and Federal guidelines at the Cleveland Lerner Research Institution and the experiments were approved by the local Institutional Animal Care and Use Committee (IACUC). Human samples were collected under IRB-approved tissue collection protocol number 3164.
Informed consent
Informed consent was obtained prior to sample collection.
Animal source
C57BL/6 (Thy1.1−), Pmel-1 transgenic (Thy1.1+Vβ13+), IFNγR1 knockout mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Cell line authentication
B16-F10 cells, derived from a gp100+ spontaneous murine melanoma cell line, were obtained from American Type Culture Collection (ATCC) (Manassas, VA). Cell culture was performed under standardized protocols to ensure that phenotypically similar cells are implanted during each experiment. In our laboratory, cell lines are routinely tested for pathogens, and expanded to produce stock aliquots frozen at the same passage.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, L., Rayman, P., Pavicic, P.G. et al. Ex vivo conditioning with IL-12 protects tumor-infiltrating CD8+ T cells from negative regulation by local IFN-γ. Cancer Immunol Immunother 68, 395–405 (2019). https://doi.org/10.1007/s00262-018-2280-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2280-3