Abstract
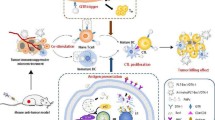
Accumulating evidence indicates that bead-based artificial antigen-presenting cells (aAPCs) are a powerful tool to induce antigen-specific T cell responses in vitro and in vivo. To date, most conventional aAPCs have been generated by coupling an antigen signal (signal 1) and one or two costimulatory signals, such as anti-CD28 with anti-LFA1 or anti-4-1BB (signal 2), onto the surfaces of cell-sized or nanoscale magnetic beads or polyester latex beads. The development of a biodegradable scaffold and the combined use of multiple costimulatory signals as well as third signals for putative clinical applications is the next step in the development of this technology. Here, a novel biodegradable aAPC platform for active immunotherapy was developed by co-encapsulating IL-2 and anti-CTLA-4 inside cell-sized polylactic-co-glycolic acid microparticles (PLGA-MPs) while co-coupling an H-2Kb/TRP2-Ig dimer and anti-CD28 onto the surface. Cytokines (activating signal) and antibodies (anti-inhibition signal) were efficiently co-encapsulated in PLGA-MP-based aAPCs and co-released without interfering with each other. The targeted, sustained co-release of IL-2 and anti-CTLA-4 achieved markedly enhanced, synergistic effects in activating and expanding tumor antigen-specific T cells both in vitro and in vivo, as well as in inhibiting tumor growth in a mouse melanoma model, as compared with conventional two-signal aAPCs and IL-2 or anti-CTLA-4 single-released aAPCs. These data revealed the feasibility and importance of the paracrine release of multiple costimulatory molecules and cytokines from biodegradable aAPCs and thus provide a proof of principle for the future use of polymeric aAPCs for active immunotherapy of tumors and infectious diseases.






Similar content being viewed by others
Abbreviations
- 7-AAD:
-
7-amino-actinomycin D
- aAPCs:
-
Artificial antigen-presenting cells
- MNPs:
-
Micro- and nanoparticles
- MPs:
-
Microparticles
- PEI:
-
Polyethylenimine
- PLGA:
-
Polylactic-co-glycolic acid
- pMHC:
-
Peptide-major histocompatibility complex
- TRP2:
-
Tyrosinase-related protein 2
References
Lee SJ, Yang A, Wu TC, Hung CF (2016) Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol 27(5):e51. doi:10.3802/jgo.2016.27.e51
Snell LM, Osokine I, Yamada DH, De la Fuente JR, Elsaesser HJ, Brooks DG (2016) Overcoming CD4 Th1 cell fate restrictions to sustain antiviral CD8 T cells and control persistent virus infection. Cell Rep 16(12):3286–3296. doi:10.1016/j.celrep.2016.08.065
Hu Z, Xia J, Fan W, Wargo J, Yang YG (2016) Human melanoma immunotherapy using tumor antigen-specific T cells generated in humanized mice. Oncotarget 7(6):6448–6459. doi:10.18632/oncotarget.7044
Osada T, Nagaoka K, Takahara M, Yang XY, Liu CX, Guo H, Roy Choudhury K, Hobeika A, Hartman Z, Morse MA, Lyerly HK (2015) Precision cancer immunotherapy: optimizing dendritic cell-based strategies to induce tumor antigen-specific T-cell responses against individual patient tumors. J Immunother 38(4):155–164. doi:10.1097/CJI.0000000000000075
Zhang N, Bevan MJ (2011) CD8(+) T cells: foot soldiers of the immune system. Immunity 35(2):161–168. doi:10.1016/j.immuni.2011.07.010
Bhargava A, Mishra D, Banerjee S, Mishra PK (2012) Dendritic cell engineering for tumor immunotherapy: from biology to clinical translation. Immunotherapy 4(7):703–718. doi:10.2217/Imt.12.40
Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG (2016) Dendritic cell-based immunotherapy: state of the art and beyond. Clin Cancer Res 22(8):1897–1906. doi:10.1158/1078-0432.CCR-15-1399
Kim JV, Latouche JB, Riviere I, Sadelain M (2004) The ABCs of artificial antigen presentation. Nat Biotechnol 22(4):403–410. doi:10.1038/nbt955
Butler MO, Hirano N (2014) Human cell-based artificial antigen-presenting cells for cancer immunotherapy. Immunol Rev 257(1):191–209. doi:10.1111/imr.12129
Eggermont LJ, Paulis LE, Tel J, Figdor CG (2014) Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells. Trends Biotechnol 32(9):456–465. doi:10.1016/j.tibtech.2014.06.007
Goto T, Nishida T, Takagi E, Miyao K, Koyama D, Sakemura R, Hanajiri R, Watanabe K, Imahashi N, Terakura S, Murata M, Kiyoi H (2016) Programmed death-ligand 1 on antigen-presenting cells facilitates the induction of antigen-specific cytotoxic T lymphocytes: application to adoptive T-cell immunotherapy. J Immunother 39(8):306–315. doi:10.1097/CJI.0000000000000136
Sun L, Guo H, Jiang R, Lu L, Liu T, Zhang Z, He X (2016) Artificial antigen-presenting cells expressing AFP(158-166) peptide and interleukin-15 activate AFP-specific cytotoxic T lymphocytes. Oncotarget 7(14):17579–17590. doi:10.18632/oncotarget.8198
Garnier A, Hamieh M, Drouet A, Leprince J, Vivien D, Frebourg T, Le Mauff B, Latouche JB, Toutirais O (2016) Artificial antigen-presenting cells expressing HLA class II molecules as an effective tool for amplifying human specific memory CD4(+) T cells. Immunol Cell Biol 94(7):662–672. doi:10.1038/icb.2016.25
Perica K, Kosmides AK, Schneck JP (2014) Linking form to function: biophysical aspects of artificial antigen presenting cell design. Biochim Biophys Acta 1853(4):781–790. doi:10.1016/j.bbamcr.2014.09.001
Bruns H, Bessell C, Varela JC, Haupt C, Fang J, Pasemann S, Mackensen A, Oelke M, Schneck JP, Schutz C (2015) CD47 enhances in vivo functionality of artificial antigen-presenting cells. Clin Cancer Res 21(9):2075–2083. doi:10.1158/1078-0432.CCR-14-2696
Perica K, Bieler JG, Schutz C, Varela JC, Douglass J, Skora A, Chiu YL, Oelke M, Kinzler K, Zhou S, Vogelstein B, Schneck JP (2015) Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano 9(7):6861–6871. doi:10.1021/acsnano.5b02829
Durai M, Krueger C, Ye Z, Cheng L, Mackensen A, Oelke M, Schneck JP (2009) In vivo functional efficacy of tumor-specific T cells expanded using HLA-Ig based artificial antigen presenting cells (aAPC). Cancer Immunol Immunother 58(2):209–220. doi:10.1007/s00262-008-0542-1
Lu XL, Jiang XB, Liu RE, Zhang SM, Liang ZH (2009) In vivo anti-melanoma efficacy of allo-restricted CTLs specific for melanoma expanded by artificial antigen-presenting cells. Cancer Immunol Immunother 58(4):629–638. doi:10.1007/s00262-008-0573-7
Chiu YL, Schneck JP, Oelke M (2011) HLA-Ig based artificial antigen presenting cells for efficient ex vivo expansion of human CTL. J Vis Exp. doi:10.3791/2801
Shen C, Cheng K, Miao S, Wang W, He Y, Meng F, Zhang J (2013) Latex bead-based artificial antigen-presenting cells induce tumor-specific CTL responses in the native T-cell repertoires and inhibit tumor growth. Immunol Lett 150(1–2):1–11. doi:10.1016/j.imlet.2013.01.003
Han FY, Thurecht KJ, Whittaker AK, Smith MT (2016) Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front Pharmacol 7:185. doi:10.3389/fphar.2016.00185
Pagels RF, Prud’homme RK (2015) Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. J Control Release 219:519–535. doi:10.1016/j.jconrel.2015.09.001
Iqbal M, Zafar N, Fessi H, Elaissari A (2015) Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm 496(2):173–190. doi:10.1016/j.ijpharm.2015.10.057
Shah SR, Henslee AM, Spicer PP, Yokota S, Petrichenko S, Allahabadi S, Bennett GN, Wong ME, Kasper FK, Mikos AG (2014) Effects of antibiotic physicochemical properties on their release kinetics from biodegradable polymer microparticles. Pharm Res 31(12):3379–3389. doi:10.1007/s11095-014-1427-y
Donat U, Rother J, Schäfer S, Hess M, Härtl B, Kober C, Langbein-Laugwitz J, Stritzker J, Chen NG, Aguilar RJ (2014) Characterization of metastasis formation and virotherapy in the human C33A cervical cancer model. PLoS One 9(6):e98533. doi:10.1371/journal.pone.0098533
Geekiyanage H, Galanis E (2016) MiR-31 and miR-128 regulates poliovirus receptor-related 4 mediated measles virus infectivity in tumors. Mol Oncol 10(9):1387–1403. doi:10.1016/j.molonc.2016.07.007
Wang W, Fang K, Li MC, Chang D, Shahzad KA, Xu T, Zhang L, Gu N, Shen CL (2016) A biodegradable killer microparticle to selectively deplete antigen-specific T cells in vitro and in vivo. Oncotarget 7(11):12176–12190. doi:10.18632/oncotarget.7519
He C, Ma H, Cheng Y, Li D, Gong Y, Liu J, Tian H, Chen X (2015) PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for localized and combined treatment of human osteosarcoma. J Control Release 213:e18. doi:10.1016/j.jconrel.2015.05.026
Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM (2011) An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. J Biol Chem 286(40):34883–34892. doi:10.1074/jbc.M111.276329
Han H, Peng JR, Chen PC, Gong L, Qiao SS, Wang WZ, Cui ZQ, Yu X, Wei YH, Leng XS (2011) A novel system of artificial antigen-presenting cells efficiently stimulates Flu peptide-specific cytotoxic T cells in vitro. Biochem Biophys Res Commun 411(3):530–535. doi:10.1016/j.bbrc.2011.06.164
Steenblock ER, Fahmy TM (2008) A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol Ther 16(4):765–772. doi:10.1038/mt.2008.11
Sunshine JC, Perica K, Schneck JP, Green JJ (2014) Particle shape dependence of CD8 + T cell activation by artificial antigen presenting cells. Biomaterials 35(1):269–277. doi:10.1016/j.biomaterials.2013.09.050
Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ (2015) Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 11(13):1519–1525. doi:10.1002/smll.201402369
Jiang T, Zhou C, Ren S (2016) Role of IL-2 in cancer immunotherapy. Oncoimmunology 5(6):e1163462. doi:10.1080/2162402X.2016.1163462
Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH (2016) Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol 34:539–573. doi:10.1146/annurev-immunol-032414-112049
Khoja L, Atenafu EG, Ye Q, Gedye C, Chappell M, Hogg D, Butler MO, Joshua AM (2016) Real-world efficacy, toxicity and clinical management of ipilimumab treatment in metastatic melanoma. Oncol Lett 11(2):1581–1585. doi:10.3892/ol.2015.4069
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81172823, 81372448) and the Science and Technology Support Program of Jiangsu Province (BE2012739).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, L., Shahzad, K.A. et al. Paracrine release of IL-2 and anti-CTLA-4 enhances the ability of artificial polymer antigen-presenting cells to expand antigen-specific T cells and inhibit tumor growth in a mouse model. Cancer Immunol Immunother 66, 1229–1241 (2017). https://doi.org/10.1007/s00262-017-2016-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2016-9