Abstract
Vδ2+ T cells are a subpopulation of γδ T cells in humans that are cytotoxic towards cells which accumulate isopentenyl pyrophosphate. The nitrogen-containing bisphosphonate, zoledronic acid (ZA), can induce tumour cell lines to accumulate isopentenyl pyrophosphate, thus rendering them more susceptible to Vδ2+ T cell cytotoxicity. However, little is known about whether ZA renders other, non-malignant cell types susceptible. In this study we focussed on macrophages (Mϕs), as these cells have been shown to take up ZA. We differentiated peripheral blood monocytes from healthy donors into Mϕs and then treated them with IFN-γ or IL-4 to generate M1 and M2 Mϕs, respectively. We characterised these Mϕs based on their phenotype and cytokine production and then tested whether ZA rendered them susceptible to Vδ2+ T cell cytotoxicity. Consistent with the literature, IFN-γ-treated Mϕs expressed higher levels of the M1 markers CD64 and IL-12p70, whereas IL-4-treated Mϕs expressed higher levels of the M2 markers CD206 and chemokine (C–C motif) ligand 18. When treated with ZA, both M1 and M2 Mϕs became susceptible to Vδ2+ T cell cytotoxicity. Vδ2+ T cells expressed perforin and degranulated in response to ZA-treated Mϕs as shown by mobilisation of CD107a and CD107b to the cell surface. Furthermore, cytotoxicity towards ZA-treated Mϕs was sensitive—at least in part—to the perforin inhibitor concanamycin A. These findings suggest that ZA can render M1 and M2 Mϕs susceptible to Vδ2+ T cell cytotoxicity in a perforin-dependent manner, which has important implications regarding the use of ZA in cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human peripheral blood contains a subpopulation of γδ T cells that express TCRs composed of Vγ9 and Vδ2 subunits. These cells—referred to here as Vδ2+ T cells—typically represent 0.5–5% of peripheral blood T cells and exert potent cytotoxicity against their target cells.
Vδ2+ T cells detect intermediates of isoprenoid biosynthesis, namely isopentenyl pyrophosphate (IPP) and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate. IPP is generated by the endogenous mevalonate pathway as well as the exogenous 1-deoxy-d-xylulose-5-phosphate pathway, whereas (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate is generated by the 1-deoxy-d-xylulose-5-phosphate pathway only [1]. The mevalonate pathway is often dysregulated in malignant and infected cells, resulting in accumulation of IPP and increased susceptibility to Vδ2+ T cell cytotoxicity [2, 3]. Moreover, certain cells accumulate IPP when exposed to the nitrogen-containing bisphosphonate (NBP), zoledronic acid (ZA) [4], a synthetic drug that inhibits an enzyme of the mevalonate pathway called farnesyl pyrophosphate synthase [5]. Although the precise mechanism of IPP and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate recognition by Vδ2+ T cells has yet to be determined, evidence suggests that it is TCR dependent and involves butyrophilin 3A1 [6].
Zoledronic acid is typically used to treat complications associated with excessive bone resorption in diseases such as osteoporosis, Paget’s disease and metastatic bone disease [7]. In terms of its mode of action, ZA binds to bone and disrupts the activity of bone remodelling cells called osteoclasts [8]. ZA also has potential as an immunotherapy for cancer, the proof of concept for which has already been demonstrated in clinical trials [9,10,11]. Although in cancer its mode of action is poorly understood, experiments in vitro have shown that tumour cell lines from a broad range of haematological and solid malignancies become more susceptible to Vδ2+ T cell cytotoxicity when exposed to ZA, suggesting a role for Vδ2+ T cells [12,13,14]. However, the capacity for ZA to induce susceptibility in other, non-malignant cell types is poorly characterised and could provide insight that helps to better understand the effects of this drug and improve its clinical application. In this study we have focussed on macrophages (denoted here as Mϕs) because these cells have been shown recently to take up NBPs in vivo [15] and are implicated in the progression of cancer [16].
Mϕs are tissue-resident phagocytic cells that play a critical role in tissue repair as well as immunity against pathogenic infection and malignant transformation [17]. Mϕs display functional plasticity that is intricately linked to their surrounding microenvironment [18]. Researchers have categorised the different functional states of Mϕs according to their capacity to either promote inflammation or suppress it. At one end of the spectrum are pro-inflammatory Mϕs, also referred to as M1 or classically activated Mϕs, and at the other end are anti-inflammatory Mϕs, also known as M2 or alternatively activated Mϕs [19]. IFN-γ and IL-4 have been identified as key drivers of these opposing M1 and M2 phenotypes, respectively [19].
As part of our ongoing studies into how ZA stimulates anti-tumour responses in Vδ2+ T cells, we identified a previously unexplored effect involving Vδ2+ T cell targeting of myeloid cells. Recently, we showed that ZA can render peripheral blood monocytes susceptible to Vδ2+ T cell cytotoxicity in vitro [20]. In a subsequent study by Junankar et al., tumour-associated Mϕs (TAMs) in breast cancer were identified as important targets for NBPs in vivo [15]. Therefore, we further explored the concept of Vδ2+ T cell targeting of myeloid cells, and found that ZA can render M1 and M2 Mϕs susceptible to Vδ2+ T cell cytotoxicity. Furthermore, we found that Vδ2+ T cell cytotoxicity towards ZA-treated Mϕs was dependent—at least in part—on perforin. This novel insight into the interplay between Vδ2+ T cells and Mϕs has important implications regarding the use of ZA in cancer immunotherapy.
Materials and methods
PBMC isolation
Anonymised leukocyte cones from healthy donors were obtained from the National Health Service blood transfusion unit at St. George’s Hospital, London. PBMCs were isolated by density-adjusted centrifugation using Histopaque-1077 (Sigma-Aldrich). RBCs were lysed with ammonium chloride solution and platelets removed by slow-speed centrifugation. PBMCs were resuspended at 2 × 107 cells/ml of freezing medium (45% RPMI-1640, 45% FBS and 10% DMSO; all from Sigma-Aldrich) and frozen at −80 °C in Mr Frosty freezing containers (Thermo Scientific) prior to transferring them to liquid nitrogen.
Cell culture
All cell culture was carried out in a humidified incubator at 37 °C with 5% CO2. To generate Mϕs, monocytes were isolated from PBMCs using CD14 microbeads according to the manufacturer’s instructions (Miltenyi Biotec). Monocytes were resuspended in serum-free medium (RPMI-1640 containing 2 mM l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin; all from Sigma-Aldrich) at a density of 3.8 × 105 cells/ml, and 200 μl, 2 or 5 ml of cell suspension added per well of 96-well, 12-well or 6-well tissue culture plates, respectively (Thermo Scientific). Monocytes were cultured for 2 h, after which time the majority of cells were adherent to the tissue culture plate. This process is known to activate monocytes and initiate the macrophage colony-stimulating factor production required for Mϕ differentiation [21]. The adherent monocytes were then cultured for 10 days in complete medium (RPMI-1640 containing 10% FBS, 2 mM l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin), after which time the monocytes had differentiated into Mϕs, as indicated by the morphological changes and plastic adherence observed using light microscopy. M1 and M2 Mϕs were generated by adding 25 ng/ml of recombinant human IFN-γ or IL-4 (R and D Systems), respectively, on day 7. Mϕs that had not been treated with IFN-γ or IL-4 (designated M0s) were used as controls throughout. 10 μM ZA (Sigma-Aldrich) was added to the Mϕs on day 9. To generate pure populations of Vδ2+ T cells, PBMCs were resuspended at 2 × 106 cells/ml of complete medium containing 1 μM ZA and 5 ng/ml recombinant human IL-2 (R and D Systems), and 250 μls of cell suspension added per well of 96-well round-bottomed tissue culture plates (Thermo Scientific). The cells were cultured for 9 days and fed every 2–3 days with fresh medium containing 5 ng/ml IL-2. Dead cells and non-γδ T cells were depleted sequentially using dead cell removal kits and TCRγδ negative isolation kits according to the manufacturer’s instructions (Miltenyi Biotec). The purity of Vδ2+CD3+ cells was assessed by flow cytometry using PE-conjugated mouse anti-human Vδ2 (clone 123R3; Miltenyi Biotec) and PerCP-conjugated mouse anti-human CD3 (clone SK7; Biolegend) or FITC-conjugated mouse anti-human CD3 (clone HIT3a; Becton–Dickinson). For one donor, a high percentage of Vδ1+CD3+ cells was detected post-isolation, and so Vδ1+ cells were depleted using allophycocyanin-conjugated recombinant human anti-Vδ1 (clone REA173; Miltenyi Biotec) and anti-allophycocyanin microbeads according to the manufacturer’s instructions (Miltenyi Biotec; supplementary Fig. 1). We speculate that this donor’s PBMCs had a particularly high percentage of Vδ1+ T cells prior to ZA and IL-2 stimulation and/or their Vδ1+ T cells underwent bystander expansion in response to ZA and IL-2.
Flow cytometry
Day 10 Mϕs in six-well tissue culture plates were washed twice in PBS (Sigma-Aldrich) and cultured for 15 min in PBS containing 0.25% trypsin (Life Technologies) and 2 mM EDTA (Sigma-Aldrich). Cells were detached by repeated pipetting and then washed in complete medium to deactivate the trypsin. Mϕs were resuspended in flow cytometry buffer (PBS with 1% BSA and 0.09% sodium azide; all from Sigma-Aldrich) containing either FITC-conjugated mouse anti-human CD64 (clone 10.1; Becton–Dickinson) or PE-conjugated mouse anti-human CD206 (clone 19.2; Becton–Dickinson). Matched isotype controls were used to determine the amount of background expression. After 10 min at room temperature, cells were washed in flow cytometry buffer and fixed in CellFIX (Becton–Dickinson). Perforin expression in Vδ2+ T cells was assessed in PBMCs cultured with ZA and IL-2 for 0, 1 and 9 days as described in "Cell culture". Cells were resuspended in flow cytometry buffer containing PE-conjugated mouse anti-human Vδ2 (clone 123R3; Miltenyi Biotec) and PerCP-conjugated mouse anti-human CD3 (clone SK7; Biolegend). After 10 min at room temperature, cells were washed in flow cytometry buffer and simultaneously fixed and permeabilised using Cytofix/Cytoperm (Becton–Dickinson) according to the manufacturer’s instructions. Cells were washed and resuspended in Perm/Wash buffer (Becton–Dickinson) and then labelled with FITC-conjugated mouse anti-human perforin (clone δG9; Becton–Dickinson) or matched isotype controls. After 10 min at room temperature, cells were washed in Perm/Wash buffer and resuspended in flow cytometry buffer. Samples were acquired on an LSR II flow cytometer (Becton–Dickinson) and analysed using FlowJo software. All comparatively analysed samples were acquired on the same day except for the time course of perforin expression where day 0, 1 and 9 samples were acquired on different days. The mean fluorescence intensity (MFI) values stated throughout are arithmetic means.
ELISAs
Day 10 Mϕs in 12-well tissue culture plates were washed twice in PBS and cultured overnight in complete medium (1 ml/well) with or without 100 ng/ml LPS (E.coli 0127:B8; Sigma-Aldrich). The concentration of IL-12p70 and chemokine (C–C motif) ligand (CCL) 18 within cell-free culture supernatants was determined using DuoSet ELISA kits according to the manufacturer’s instructions (R and D Systems). Optical densities at 450 nm were determined using a microplate reader (Dynex), and concentrations were extrapolated from standard curve data using a four parameter logistic model generated by GraphPad Prism 6 (GraphPad Software). Standard curves were 31.25–2000 pg/ml for IL-12p70, and 7.8125–500 pg/ml for CCL18.
Carboxyfluorescein succinimidyl ester/Zombie-NIR cytotoxicity assay
Detaching the Mϕs from the tissue culture plates prior to performing the cytotoxicity assays resulted in poor viability; therefore, cytotoxicity was assessed by adding Vδ2+ T cells directly to adherent Mϕs. Day 10 Mϕs in 12-well tissue culture plates were washed twice in PBS and then cultured for 20 min in PBS containing 1 μM carboxyfluorescein succinimidyl ester (CFSE; Life Technologies). Mϕs were washed three times in complete medium and then cultured overnight with or without 1.52 × 106 autologous Vδ2+ T cells per well in 2 ml complete medium to obtain an E:T ratio of 2:1 based on the initial seeding density of monocytes. For some experiments Vδ2+ T cells were pre-treated for 2 h with or without 100 ng/ml concanamycin A (CMA; Abcam) or DMSO, then washed three times in complete medium prior to being cultured with Mϕs. Non-adherent cells were collected and adherent cells detached from the tissue culture plates as described in “Flow cytometry”. All cells were washed in PBS and then labelled with Zombie-NIR live/dead cell discrimination dye according to the manufacturer’s instructions (Biolegend). Zombie-NIR binds to amine groups on proteins, but does not penetrate an intact plasma membrane. Live cells have relatively low expression because only cell surface proteins are available for binding, whereas dead cells exhibit higher levels of expression because their compromised plasma membrane permits binding to both extracellular and intracellular proteins. After 15 min at room temperature, cells were washed in complete medium and fixed in CellFIX. Samples were acquired on an LSR II flow cytometer and analysed using FlowJo software. All comparatively analysed samples were acquired on the same day.
CD107 mobilisation assay
Day 10 Mϕs in 96-well tissue culture plates were washed three times in PBS and then cultured for 5 h with 1.52 × 105 autologous Vδ2+ T cells per well in 200 μl complete medium to obtain an E:T ratio of 2:1 based on the initial seeding density of monocytes. Allophycocyanin-conjugated mouse anti-human CD107a (clone H4A3; Biolegend) and FITC-conjugated mouse anti-human CD107b (clone H4B4; Biolegend) or matched isotype controls were added directly to the wells at the start of the co-culture along with 1 μg/ml of monensin to neutralise intracellular acidity. Cells were then collected and labelled with PE-conjugated mouse anti-human Vδ2 (clone 123R3; Miltenyi Biotec) and PerCP-conjugated mouse anti-human CD3 (clone SK7; Biolegend) as described in “Flow cytometry”. Samples were acquired on an LSR II flow cytometer and analysed using FlowJo software. All comparatively analysed samples were acquired on the same day.
Statistical analyses
Data in Figs. 1b, c, 3b, d and 4c were analysed by repeated measures one-way or two-way ANOVA and comparisons between means carried out using either Tukey’s or Sidak’s multiple comparison tests (GraphPad Prism 6). *, **, *** and **** were used to indicate p values of <0.05, <0.01, <0.001 and <0.0001, respectively. Gaussian distributions were assumed. Data in Fig. 2b included a three-way (3 × 2 × 2) factorial design repeated six times using cells from six different donors. The three factors were Mϕ type (M0, M1 and M2), ±ZA and ±Vδ2 cells. Data in Fig. 4b were a three-way (3 × 2 × 4) factorial design repeated five times using cells from five different donors. The three factors were Mϕ type (M0, M1 and M2), ±ZA and ±Vδ2 cells (−Vδ2, +Vδ2, +Vδ2[DMSO] and +Vδ2[CMA]). Data in Figs. 2b and 4b were analysed by three-way ANOVA and comparisons between means carried out using Fisher’s least significant difference (LSD; Genstat 18). Assumptions underlying the analysis were checked using the diagnostic plots produced by the software. LSDs at the 5, 1 and 0.1% level are depicted by black intervals, and differences in the means that were greater than this interval were deemed significant to an equivalent p value of <0.05, <0.01 and <0.001, respectively.
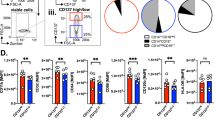
Characterisation of M1 and M2 Mϕs treated with or without ZA. a, b Flow cytometry was used to measure the expression of CD64 and CD206 on M0, M1 and M2 Mϕs treated with (orange) or without (blue) ZA for the last 18 h of culture. a Representative flow cytometry plots from one of six donors (−ZA). Dead cells and debris were excluded based on forward scatter (FSC) and side scatter (SSC) using gate (G) 1. Unfilled overlays = test, filled overlays = isotype. Numbers on the plots are percentage of cells within the marker. b Individual data points and means for six donors. Test MFIs for the total G1 population were divided by isotype controls to obtain relative MFIs. c M0, M1 and M2 Mϕs treated with (orange) or without (blue) ZA for the last 18 h of culture were cultured overnight in fresh medium with or without 100 ng/ml LPS. The concentration of IL-12p70 and CCL18 in culture supernatants was measured using ELISAs. Data for IL-12p70 is in the presence of LPS, whereas data for CCL18 is in the absence of LPS. Individual data points and means for six donors are shown. For b and c, data were analysed by repeated measures two-way ANOVA and comparisons between means carried out using Tukey’s multiple comparison tests. *** and **** indicate p values of <0.001 and <0.0001, respectively. Statistical differences for comparisons within the +ZA (orange) data sets are not shown
Vδ2+ T cell cytotoxicity towards ZA-treated M1 and M2 Mϕs. M0, M1 and M2 Mϕs treated with or without ZA for the last 18 h of culture were labelled with CFSE and cultured overnight with or without autologous Vδ2+ T cells. Flow cytometry was then used to measure Zombie-NIR expression. a Representative flow cytometry contour plots from one of six donors showing the gating strategy used to determine Zombie-NIR expression in M0 Mϕs. Mϕs were gated based on FSC and SSC using G1. CFSE+ cells within G1 were gated using G2. The percentage of Zombie-NIRhigh cells (i.e. dead cells) within G1 + G2 was then determined using G3. Numbers on the contour plots are percentages of cells within G3. b Individual data points and means for six donors. Data were analysed by three-way ANOVA and comparisons between means carried out using Fisher’s LSD tests. The 5, 1 and 0.1% LSDs are depicted by the black intervals
Results
ZA did not alter M1 or M2 markers on Mϕs
We differentiated monocytes from the peripheral blood of healthy donors into Mϕs and treated them with IFN-γ or IL-4 to generate M1 and M2 Mϕs, respectively. We then characterised these Mϕs based on their expression of markers for M1 Mϕs (CD64 and IL-12p70) and M2 Mϕs (CD206 and CCL18) [19, 22]. M1 Mϕs had upregulated expression of CD64, whereas M2 Mϕs had downregulated CD64 and upregulated CD206 (Fig. 1a, b). Although, statistically, we observed significantly higher levels of CD206 on M1 Mϕs compared with M0 Mϕs in terms of percentage expression, this was not consistent for all donors and not statistically significant in terms of relative MFI (Fig. 1b). Mϕs were then cultured overnight with or without LPS to measure the production of IL-12p70 and CCL18, respectively. M1 Mϕs produced more IL-12p70 than M0 and M2 Mϕs, whereas M2 Mϕs produced more CCL18 than M0 and M1 Mϕs (Fig. 1c). We also tested whether ZA—added for the last 18 h of culture—had any effect on these markers and found little or no difference between untreated and ZA-treated Mϕs (Fig. 1). Taken together, these data validate our protocol for generating M1 and M2 Mϕs and show that ZA does not alter the M1 and M2 profile of the Mϕs in this system.
ZA-rendered M1 and M2 Mϕs susceptible to Vδ2+ T cell cytotoxicity
To obtain sufficient cell numbers for cytotoxicity assays, we stimulated Vδ2+ T cell expansion prior to isolation. Vδ2+ T cell expansion was observed in PBMCs treated with ZA and IL-2 for 9 days, as shown by increased frequencies of Vδ2+CD3+ cells (supplementary Fig. 2). Vδ2+ T cells were purified by sequentially depleting dead cells and non-γδ T cells (mean ± standard deviation (SD) for the percentage of Vδ2+CD3+ cells from four donors = 97.2 ± 1.8; supplementary Fig. 2). The percentage of Vδ2+CD3+ cells at day 0 and day 9 pre-depletion of dead cells and non-γδ T cells was not assessed routinely; however, purities at day 9 post-depletion were assessed for all isolations performed in this study (mean ± SD for 14 isolations = 97.7 ± 1.8). We conducted preliminary experiments to determine the optimal E:T ratio and ZA concentration for Vδ2+ T cell-mediated cytotoxicity against ZA-treated Mϕs (supplementary Fig. 3). These experiments showed Vδ2+ T cell cytotoxicity and degranulation against Mϕs treated with 10 μM, but not 1 μM ZA (supplementary Fig. 3). Furthermore, they showed marked killing at the lowest E:T ratio of 2:1 (supplementary Fig. 3). Using the 10 μM concentration of ZA and 2:1 E:T ratio, we found that ZA had little or no effect on Mϕ viability in the absence of Vδ2+ T cells, and Vδ2+ T cells did not induce cell death in Mϕs that had not been treated with ZA (Fig. 2a, b). However, there was a marked increase in the amount of cell death in Mϕs that were pre-treated with ZA and then cultured with Vδ2+ T cells (Fig. 2a, b). Although, statistically, Vδ2+ T cell-mediated killing of ZA-treated M1 Mϕs was significantly higher than that of M0 Mϕs, the difference was relatively small and no statistically significant difference was found between M1 and M2 Mϕs (Fig. 2b). These results suggest that Vδ2+ T cells are cytotoxic towards ZA-treated Mϕs irrespective of their M0, M1 and M2 phenotype.
Vδ2+ T cells expressed perforin and degranulated when cultured with ZA-treated Mϕs
Perforin has been shown previously to play a role in γδ T cell cytotoxicity towards tumour cell lines [23, 24]; therefore, we tested whether perforin contributes to Vδ2+ T cell cytotoxicity towards ZA-treated Mϕs. We measured perforin expression by Vδ2+ T cells before, during and after expansion with ZA and IL-2. We found that, although resting Vδ2+ T cells expressed little or no perforin, it was markedly upregulated after 1 day of culture with ZA and IL-2 (Fig. 3a, b). After 9 days of culture with ZA and IL-2, perforin was downregulated but still expressed by Vδ2+ T cells (Fig. 3a, b). We also measured perforin expression in expanded and isolated Vδ2+ T cells from six donors and found consistent expression in terms of percentage expression (mean ± SD = 1.2 ± 0.5 vs. 23.9 ± 7.6 for isotype and test, respectively) and MFI (mean ± SD = 227.8 ± 15.2 vs. 490.7 ± 104.6 for isotype and test, respectively). To determine if Vδ2+ T cells release perforin when cultured with ZA-treated Mϕs, we measured the mobilisation of lysosomal-associated membrane protein 1 and 2 (i.e. CD107a and CD107b) to the surface of Vδ2+ T cells. CD107a—and to a lesser extent CD107b—was expressed on Vδ2+ T cells that were isolated from PBMCs after 9 days of culture with ZA and IL-2 (Fig. 3c). This may represent residual CD107 expression from the monocyte-dependent degranulation that is induced when PBMCs are exposed to ZA [20]. Vδ2+ T cells upregulated expression of CD107a and CD107b on their cell surface when cultured with ZA-treated Mϕs compared with untreated Mϕs (Fig. 3c, d). These data suggest that Vδ2+ T cells express perforin and degranulate in response to ZA-treated Mϕs, thus implicating a role for perforin in Vδ2+ T cell cytotoxicity towards ZA-treated Mϕs.
Expression of perforin and mobilisation of CD107a and CD107b in Vδ2+ T cells. a, b Flow cytometry was used to measure the expression of perforin by Vδ2+ T cells in PBMCs cultured with ZA and IL-2 for 0, 1 and 9 days. a Representative flow cytometry plots from one of three donors showing perforin expression in Vδ2+ T cells. Lymphocytes were gated based on FSC and SSC using G1. Note that G1 was extended at day 9 to incorporate blast cells. Vδ2+CD3+ cells within G1 were gated using G2. Percentage expression and MFI of perforin within G1 + G2 was then assessed. Unfilled overlays = test, filled overlays = isotype. Numbers on the histogram plots are percentage of cells within the marker. b Mean ± SD for three donors. Test MFIs for the total G1 + G2 population were divided by the isotype controls to obtain relative MFIs. Data were analysed by repeated measures one-way ANOVA and comparisons between means carried out using Tukey’s multiple comparison tests. c, d Vδ2+ T cells were cultured with or without autologous M0, M1 or M2 Mϕs that had been treated with or without ZA for the last 18 h of culture. Flow cytometry was used to measure the expression of CD107a and CD107b by Vδ2+ T cells. c Representative flow cytometry contour plots from one of three donors showing CD107a and CD107b expression on gated Vδ2+CD3+ cells. Lymphocytes were gated based on FSC and SSC using G1. Vδ2+CD3+ cells within G1 were gated using G2. Percentage expression of CD107a and CD107b within G1 + G2 was then assessed. Quadrants were set against the Vδ2 alone controls, and separate quadrants were generated for isotype and test. Numbers are percentages of cells contained within the upper right quadrants. d Individual data points and means for three donors. Data were analysed by repeated measures two-way ANOVA and comparisons between means carried out using Sidak’s multiple comparison tests. For b and d, *, **, *** and **** indicate p values of <0.05, <0.01, <0.001 and <0.0001, respectively
Vδ2+ T cell cytotoxicity towards ZA-treated Mϕs was sensitive to concanamycin A
To explore further the potential role of perforin in Vδ2+ T cell cytotoxicity towards ZA-treated Mϕs, we repeated the cytotoxicity assays shown in Fig. 2, but this time pre-treated Vδ2+ T cells with the H+-ATPase inhibitor CMA. CMA blocks acidification of cytolytic granules, which inhibits perforin-mediated but not Fas ligand-mediated cytotoxicity [25]. We found that pre-treating Vδ2+ T cells with CMA reduced their cytotoxicity towards Mϕs compared with DMSO controls (Fig. 4a, b). We calculated the percentage inhibition for Vδ2+ T cell cytotoxicity towards M0, M1 and M2 Mϕs and found that Vδ2+ T cell cytotoxicity towards M0 Mϕs was more sensitive to CMA than towards M1 Mϕs (Fig. 4c). To determine whether CMA had an effect on Vδ2+ T cell viability, we applied a gate to CFSE− cells and calculated the percentage of Zombie-NIRlow cells (supplementary Fig. 4a). There was a discernible reduction in Vδ2+ T cell viability in the presence of ZA-treated Mϕs compared with untreated Mϕs; however, there was little or no difference in Vδ2+ T cell viability between the CMA and DMSO treatment groups (supplementary Fig. 4b). These findings suggest that Vδ2+ T cell cytotoxicity towards ZA-treated Mϕs is sensitive—at least in part—to CMA, thus implicating a role for perforin.
The effect of concanamycin A on Vδ2+ T cell cytotoxicity towards ZA-treated Mϕs. M0, M1 and M2 Mϕs treated with or without ZA for the last 18 h of culture were labelled with CFSE and then cultured overnight with or without autologous Vδ2+ T cells that had been pre-treated for 2 h with or without CMA (100 ng/ml) or DMSO. Flow cytometry was then used to measure Zombie-NIR expression. a Representative flow cytometry contour plots for M0 Mϕs from one of five donors. The percentage of Zombie-NIRhigh cells (i.e. dead cells) within CFSE+ Mϕs was determined using the G1 + G2 + G3 gating strategy described in Fig. 2. Numbers on the plots are percentages of cells within G3. b Individual data points and means for five donors. Data were analysed by three-way ANOVA and comparisons between means carried out using Fisher’s LSD tests. The 0.1% LSD is depicted by the black interval. c Data in b was expressed as percentage inhibition. Within the +ZA data sets, the percentage of dead Mϕs in the absence of Vδ2+ T cells (i.e. background cell death) was subtracted from that induced by the DMSO- and CMA-treated Vδ2+ T cells. The corrected values for Mϕ cell death induced by CMA-treated Vδ2+ T cells were then expressed as a percentage of the corrected values for Mϕ cell death induced by DMSO-treated Vδ2+ T cells. These values were then converted to percentage inhibition by subtracting them from 100%. Data were analysed by repeated measures one-way ANOVA and comparisons between means carried out using Tukey’s multiple comparison tests. ** Indicates a p value <0.01
Discussion
Vδ2+ T cells in the peripheral blood of humans are regarded as sentinels against infection [26] and malignant transformation [27]. They express the inflammatory homing receptors chemokine (C–C motif) receptor 5 and chemokine (C–X–C motif) receptor 3 [28] and thus infiltrate sites of infection [29] as well as the inflammatory microenvironment of diseased tissues such as tumours [30, 31]. Mϕs are abundant in these tissues and are likely to interact closely with infiltrating Vδ2+ T cells. We explored the potential interaction between Vδ2+ T cells and Mϕs in vitro and found that ZA can render M1 and M2 Mϕs susceptible to Vδ2+ T cell cytotoxicity in a perforin-dependent manner.
Zoledronic acid has a high affinity for hydroxyapatite [32] and thus binds rapidly to bone following i.v. infusion [33]. Therefore, the Mϕs most likely to be exposed to ZA are those associated with bone and/or the surrounding tissues: for example, the TAMs in bone-related cancers such as osteosarcoma, myeloma and secondary bone metastases associated with cancers of the prostate, lung and breast. Following i.v. infusion, NBPs may also reach tissues other than bone. Intravital imaging in a murine model of breast cancer showed that a fluorescently labelled NBP—given by i.v. injection—leaked from the vasculature of mammary tumours and bound rapidly to granular microcalcifications, which were subsequently engulfed by TAMs [15]. The NBP was not retained in cells other than Mϕs, nor was it retained in B16 tumours, which lack microcalcifications [15]. This study suggests that calcified tissues other than bone can also accumulate NBPs [15]. The lack of cytotoxicity and degranulation at 1 μM ZA that was observed in our preliminary optimisation experiments suggests that the Mϕs most likely to be targeted by Vδ2+ T cells following ZA treatment are those associated with calcified tissues where the drug is likely to accumulate, which has important implications regarding the in vivo effects of this drug. It is worth noting that uptake of ZA by Mϕs in vivo may be markedly different using other methods of delivery such as liposome or nanoparticle encapsulation [34, 35] and localised injection. At the cellular level, experiments conducted in vitro suggest that ZA is taken up by myeloid cells such as monocytes, Mϕs and osteoclasts via the process of fluid phase endocytosis [36, 37].
Zoledronic acid inhibits farnesyl pyrophosphate synthase of the mevalonate pathway, which has been shown in vitro to induce apoptosis directly in the murine Mϕ-like cell line J774.2 [38]. A potential mechanism for this effect is accumulation of the pro-apoptotic analogue of ATP, Apppl, which has been reported to accumulate in ZA-treated cells such as osteoclasts and MCF-7 cells [4]. Interestingly, ZA did not affect the viability of Mϕs in our experiments; however, we used relatively short exposure times and did not look at markers of early stage apoptosis such as surface expression of phosphatidyl serine. Inhibition of farnesyl pyrophosphate synthase may also modulate the differentiation and function of Mϕs. For example, when monocyte-derived M2 Mϕs were differentiated in the presence of ZA, they had reduced expression of CD206 and IL-10, and an impaired capacity to promote angiogenesis and tumour cell invasion [39]. ZA also inhibited tumour growth in a murine model of cervical cancer, which correlated with reduced angiogenesis and decreased production of matrix metallopeptidase 9 by Mϕs proximal to and associated with tumours [40]. Furthermore, ZA reduced the onset and growth of tumours in a murine model of breast cancer, which correlated with reduced vascularisation of the tumour, reduced numbers of TAMs, and repolarisation of TAMs from an M2 to M1 phenotype [41]. Taken together, these studies suggest that ZA can modulate the differentiation of Mϕs towards an M1 phenotype. To the best of our knowledge, Vδ2+ T cell targeting of ZA-treated M1 and M2 Mϕs—as suggested by our data—has been previously unreported and broadens our understanding of the effects of ZA on Mϕs. Importantly, mice do not develop the Vδ2+ T cell subset that responds to ZA-induced accumulation of IPP because they lack the gene for butyrophilin 3A1 [42], thus highlighting the importance of using human cells for this study.
Our data suggests that ZA has the potential to kill M1 and M2 Mϕs indirectly within tissues that are exposed to the drug and infiltrated by Vδ2+ T cells. Tumours contain an abundant population of Mϕs, which typically express M2 markers and correlate with a poor prognosis [43]. In breast cancer, CCL18 production by TAMs promotes angiogenesis and thus supports tumour growth and dissemination [44]. Furthermore, M2 Mϕs in the bone marrow of multiple myeloma patients have been shown to protect malignant cells from chemotherapy-induced apoptosis [45, 46]. In contrast, osteosarcomas can contain relatively high percentages of M1 Mϕs, which are associated with reduced metastases and improved survival [47]. The potential for ZA to render Mϕs susceptible to Vδ2+ T cells may be beneficial or detrimental depending on which type of Mϕ is present in the tumour. For example, it may be beneficial in patients with breast cancer or myeloma and could explain the promising responses to ZA reported for clinical trials in these cancer types [48, 49], whereas it may be counterproductive in osteosarcoma.
It is important to note that our study has focussed on the killing capacity of activated Vδ2+ T cells. Although it would be interesting to compare the cytotoxicity of resting and activated Vδ2+ T cells, the relatively low frequency of Vδ2+ T cells in peripheral blood meant that we were unable to isolate the number of resting Vδ2+ T cells required to perform the cytotoxicity assays used in this study. Whether or not i.v. infusion of ZA—combined with i.v. or s.c. IL-2—can activate peripheral blood Vδ2+ T cells in vivo is a point of contention. Current hypotheses state that peripheral blood monocytes take up ZA following i.v. infusion and subsequently activate Vδ2+ T cells [37]; indeed, proliferation and/or differentiation of peripheral blood Vδ2+ T cells has been reported in some patients receiving ZA and IL-2 [9,10,11]. However, Vδ2+ T cell responses were not observed in all patients [50] and it is unclear whether this is due to lack of activation or detection. Importantly, Vδ2+ T cells that are pre-activated may be more cytotoxic than resting, and thus ZA-induced targeting of Mϕs by Vδ2+ T cells in vivo may be suboptimal in patients for whom ZA and IL-2 treatment fails to activate their circulating Vδ2+ T cells, thus highlighting the importance of effective Vδ2+ T cell priming in the periphery.
In our study, Vδ2+ T cell cytotoxicity towards M0, M1 and M2 Mϕs was sensitive—at least in part—to the perforin inhibitor CMA, thus implicating a role for perforin [25]. Interestingly, CMA did not inhibit cytotoxicity completely, and the degree of inhibition varied between the different types of Mϕ; specifically, Vδ2+ T cell cytotoxicity towards M0 Mϕs was more sensitive to CMA than towards M1 Mϕs. If, in our assays, CMA blocked perforin completely, our data would suggest that other mechanisms of cell-mediated cytotoxicity are involved and that the contribution of perforin versus other mechanisms of cytotoxicity varies between the different types of Mϕ. Indeed, Vδ2+ T cells have been shown to kill target cells through the expression of Fas ligand and TRAIL [51]. However, if perforin blockade was incomplete, the variation in sensitivity to CMA that was observed between the different types of Mϕ could also be attributed to differences in their susceptibility to perforin-mediated killing under conditions of suboptimal perforin activity. Nonetheless, our data suggest that perforin plays a role, which provides a useful mechanistic marker for exploring this concept in vivo.
In conclusion, this study sheds light on a potential interaction between Vδ2+ T cells and Mϕs following ZA treatment and suggests a mechanism of action for this drug that may help its future development in cancer immunotherapy.
Abbreviations
- CCL:
-
Chemokine (C–C motif) ligand
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- CMA:
-
Concanamycin A
- FSC:
-
Forward scatter
- G:
-
Gate
- IPP:
-
Isopentenyl pyrophosphate
- LSD:
-
Least significant difference
- Mϕ:
-
Macrophage
- MFI:
-
Mean (arithmetic) fluorescence intensity
- NBP:
-
Nitrogen-containing bisphosphonate
- SD:
-
Standard deviation
- SSC:
-
Side scatter
- TAM:
-
Tumour-associated macrophage
- ZA:
-
Zoledronic acid
References
Morita CT, Jin C, Sarikonda G, Wang H (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215:59–76
Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G (2003) Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 197:163–168
Kistowska M, Rossy E, Sansano S, Gober HJ, Landmann R, Mori L, De Libero G (2008) Dysregulation of the host mevalonate pathway during early bacterial infection activates human TCR gamma delta cells. Eur J Immunol 38:2200–2209
Raikkonen J, Crockett JC, Rogers MJ, Monkkonen H, Auriola S, Monkkonen J (2009) Zoledronic acid induces formation of a pro-apoptotic ATP analogue and isopentenyl pyrophosphate in osteoclasts in vivo and in MCF-7 cells in vitro. Br J Pharmacol 157:427–435
Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russell RG, Oppermann U (2006) The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci USA 103:7829–7834
Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, Li J, Kuball J, Adams EJ, Netzer S, Dechanet-Merville J, Leger A, Herrmann T, Breathnach R, Olive D, Bonneville M, Scotet E (2012) Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 120:2269–2279
Lipton A (2005) New therapeutic agents for the treatment of bone diseases. Expert Opin Biol Ther 5:817–832
Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ (2001) Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone 28:465–473
Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC (2007) Targeting human gamma delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 67:7450–7457
Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D’Asaro M, Orlando V, Scarpa F, Roberts A, Caccamo N, Stassi G, Dieli F, Hayday AC (2010) In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 161:290–297
Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J, Becker J, Schmidt-Wolf IG, Einsele H, Wilhelm M (2012) Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother 35:205–213
Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, Kabelitz D, Wesch D (2007) Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol 66:320–328
Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintiere C, Daniel P, Genetet N, Meunier B, Dupont-Bierre E, Boudjema K, Catros V (2008) Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors. Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother 57:531–539
Saitoh A, Narita M, Watanabe N, Tochiki N, Satoh N, Takizawa J, Furukawa T, Toba K, Aizawa Y, Shinada S, Takahashi M (2008) Anti-tumor cytotoxicity of gammadelta T cells expanded from peripheral blood cells of patients with myeloma and lymphoma. Med Oncol 25:137–147
Junankar S, Shay G, Jurczyluk J, Ali N, Down J, Pocock N, Parker A, Nguyen A, Sun S, Kashemirov B, McKenna CE, Croucher PI, Swarbrick A, Weilbaecher K, Phan TG, Rogers MJ (2015) Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov 5:35–42
Mills CD, Lenz LL, Harris RA (2016) A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res 76:513–516
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A et al (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20
Fowler DW, Copier J, Dalgleish AG, Bodman-Smith MD (2014) Zoledronic acid causes gammadelta T cells to target monocytes and down-modulate inflammatory homing. Immunology 143:539–549
Wahl LM, Wahl SM, Smythies LE, Smith PD (2006) Isolation of human monocyte populations. Curr Protoc Immunol. Chapter 7: Unit 7.6A
Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, Tak PP, Baeten DL (2012) Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods 375:196–206
Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, Pauza CD, Strome SE, Chapoval AI (2008) Isopentenyl pyrophosphate-activated CD56 + gamma delta T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res 14:4232–4240
Mattarollo SR, Kenna T, Nieda M, Nicol AJ (2007) Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother 56:1285–1297
Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K (1996) Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol 156:3678–3686
Dieli F, Troye-Blomberg M, Farouk SE, Sireci G, Salerno A (2001) Biology of gammadelta T cells in tuberculosis and malaria. Curr Mol Med 1:437–446
Fowler DW, Bodman-Smith MD (2015) Harnessing the power of Vdelta2 cells in cancer immunotherapy. Clin Exp Immunol 180:1–10
Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D (2002) Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of V delta 2/V gamma 9 gamma delta T cells. J Immunol 168:4920–4929
Liuzzi AR, Kift-Morgan A, Lopez-Anton M, Friberg IM, Zhang J, Brook AC, Roberts GW, Donovan KL, Colmont CS, Toleman MA, Bowen T, Johnson DW, Topley N, Moser B, Fraser DJ, Eberl M (2016) Unconventional human T cells accumulate at the site of infection in response to microbial ligands and induce local tissue remodeling. J Immunol 197:2195–2207
Cordova A, Toia F, La Mendola C, Orlando V, Meraviglia S, Rinaldi G, Todaro M, Cicero G, Zichichi L, Donni PL, Caccamo N, Stassi G, Dieli F, Moschella F (2012) Characterization of human gammadelta T lymphocytes infiltrating primary malignant melanomas. PLoS ONE 7:e49878
Bank I, Book M, Huszar M, Baram Y, Schnirer I, Brenner H (1993) V delta 2 + gamma delta T lymphocytes are cytotoxic to the MCF 7 breast carcinoma cell line and can be detected among the T cells that infiltrate breast tumors. Clin Immunol Immunopathol 67:17–24
Lawson MA, Xia Z, Barnett BL, Triffitt JT, Phipps RJ, Dunford JE, Locklin RM, Ebetino FH, Russell RG (2010) Differences between bisphosphonates in binding affinities for hydroxyapatite. J Biomed Mater Res B Appl Biomater 92:149–155
Kimmel DB (2007) Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res 86:1022–1033
Marra M, Salzano G, Leonetti C, Porru M, Franco R, Zappavigna S, Liguori G, Botti G, Chieffi P, Lamberti M, Vitale G, Abbruzzese A, La Rotonda MI, De Rosa G, Caraglia M (2012) New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol Adv 30:302–309
Schiraldi C, Zappavigna S, D’Agostino A, Porto S, Gaito O, Lusa S, Lamberti M, De Rosa M, De Rosa G, Caraglia M (2014) Nanoparticles for the delivery of zoledronic acid to prostate cancer cells: a comparative analysis through time lapse video-microscopy technique. Cancer Biol Ther 15:1524–1532
Thompson K, Rogers MJ, Coxon FP, Crockett JC (2006) Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol 69:1624–1632
Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K (2009) Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol 144:245–250
Rogers TL, Wind N, Hughes R, Nutter F, Brown HK, Vasiliadou I, Ottewell PD, Holen I (2013) Macrophages as potential targets for zoledronic acid outside the skeleton-evidence from in vitro and in vivo models. Cell Oncol (Dordr) 36:505–514
Comito G, Segura CP, Taddei ML, Lanciotti M, Serni S, Morandi A, Chiarugi P, Giannoni E (2017) Zoledronic acid impairs stromal reactivity by inhibiting M2-macrophages polarization and prostate cancer-associated fibroblasts. Oncotarget 8:118–132
Giraudo E, Inoue M, Hanahan D (2004) An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114:623–633
Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Monkkonen H, Boccadoro M, Forni G, Musiani P, Bosia A, Cavallo F, Massaia M (2010) Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med 14:2803–2815
Karunakaran MM, Gobel TW, Starick L, Walter L, Herrmann T (2014) Vgamma9 and Vdelta2 T cell antigen receptor genes and butyrophilin 3 (BTN3) emerged with placental mammals and are concomitantly preserved in selected species like alpaca (Vicugna pacos). Immunogenetics 66:243–254
Schmieder A, Michel J, Schonhaar K, Goerdt S, Schledzewski K (2012) Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol 22:289–297
Lin L, Chen YS, Yao YD, Chen JQ, Chen JN, Huang SY, Zeng YJ, Yao HR, Zeng SH, Fu YS, Song EW (2015) CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget 6:34758–34773
Beider K, Bitner H, Leiba M, Gutwein O, Koren-Michowitz M, Ostrovsky O, Abraham M, Wald H, Galun E, Peled A, Nagler A (2014) Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget 5:11283–11296
Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q (2009) Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 114:3625–3628
Buddingh EP, Kuijjer ML, Duim RA, Burger H, Agelopoulos K, Myklebost O, Serra M, Mertens F, Hogendoorn PC, Lankester AC, Cleton-Jansen AM (2011) Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res 17:2110–2119
Aviles A, Neri N, Huerta-Guzman J, Nambo MJ (2013) Randomized clinical trial of zoledronic acid in multiple myeloma patients undergoing high-dose chemotherapy and stem-cell transplantation. Curr Oncol 20:e13–e20
Valachis A, Polyzos NP, Coleman RE, Gnant M, Eidtmann H, Brufsky AM, Aft R, Tevaarwerk AJ, Swenson K, Lind P, Mauri D (2013) Adjuvant therapy with zoledronic acid in patients with breast cancer: a systematic review and meta-analysis. Oncologist 18:353–361
Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G, Liu G, Eickhoff JC, McNeel DG, Malkovsky M (2011) Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother 60:1447–1460
Li H, Xiang Z, Feng T, Li J, Liu Y, Fan Y, Lu Q, Yin Z, Yu M, Shen C, Tu W (2013) Human Vgamma9Vdelta2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells. Cell Mol Immunol 10:159–164
Acknowledgements
The authors would like to thank the Institute for Cancer Vaccines and Immunotherapy (Registered Charity Number 1080343) for funding this research. The authors would like to thank Dr. David Lovell, Reader in Medical Statistics at St. George’s University of London, for his advice and expertise in performing the statistical analyses using ANOVA for the three-way factorial design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fowler, D.W., Copier, J., Dalgleish, A.G. et al. Zoledronic acid renders human M1 and M2 macrophages susceptible to Vδ2+ γδ T cell cytotoxicity in a perforin-dependent manner. Cancer Immunol Immunother 66, 1205–1215 (2017). https://doi.org/10.1007/s00262-017-2011-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2011-1