Abstract
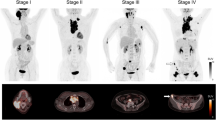
[F-18]-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) is a non-invasive imaging technique which has recently been validated for the assessment of therapy response in patients with aggressive non-Hodgkin’s lymphoma. Our objective was to determine its value for the evaluation of immunotherapy efficacy in immunocompetent Balb/c mice injected with the A20 syngeneic B lymphoma cell line. The high level of in vitro FDG uptake by A20 cells validated the model for further imaging studies. When injected intravenously, the tumour developed as nodular lesions mostly in liver and spleen, thus mimicking the natural course of an aggressive human lymphoma. FDG-PET provided three-dimensionnal images of tumour extension including non-palpable lesions, in good correlation with ex vivo macroscopic examination. When mice were pre-immunized with an A20 cell lysate in adjuvant before tumour challenge, their significantly longer survival, compared to control mice, were associated with a lower incidence of lymphoma visualized by PET at different time points. Estimation of tumour growth and metabolism using the calculated tumour volumes and maximum standardized uptake values, respectively, also demonstrated delayed lymphoma development and lower activity in the vaccinated mice. Thus, FDG-PET is a sensitive tool relevant for early detection and follow-up of internal tumours, allowing discrimination between treated and non-treated small animal cohorts without invasive intervention.






Similar content being viewed by others
References
Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, Gaulard P, Garderet L, Lepage E, Reyes F, Meignan M (2005) [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 105:1376–1381
Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Thomas J, de Groot T, Balzarini J, De Wolf-Peeters C, Mortelmans L, Verhoef G (2002) Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol 13:1356–1363
Juweid ME, Cheson BD (2005) Role of positron emission tomography in lymphoma. J Clin Oncol 23:4577–4580
Czernin J, Weber WA, Herschman HR (2006) Molecular imaging in the development of cancer therapeutics. Annu Rev Med 57:99–118
Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H (1990) Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun 170:223–230
Ido T, Wan CN, Casella JS et al (1978) Labeled 2-deoxy-d-glucose analogs: 18F labeled 2-deoxy-2-fluoro-d-glucose, 2-deoxy-2-fluoro-d-mannose and 14C-2-deoxy-2-fluoro-d-glucose. J Labeled Comp Radiopharm 14:175–183
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Burton C, Ell P, Linch D (2004) The role of PET imaging in lymphoma. Br J Haematol 126:772–784
Abbey CK, Borowsky AD, McGoldrick ET, Gregg JP, Maglione JE, Cardiff RD, Cherry SR (2004) In vivo positron-emission tomography imaging of progression and transformation in a mouse model of mammary neoplasia. Proc Natl Acad Sci USA 101:11438–11443
Lyons SK (2005) Advances in imaging mouse tumour models in vivo. J Pathol 205:194–205
Briones J, Timmerman J, Levy R (2002) In vivo antitumor effect of CD40L-transduced tumor cells as a vaccine for B-cell lymphoma. Cancer Res 62:3195–3199
Levitsky HI, Montgomery J, Ahmadzadeh M, Staveley-O’Carroll K, Guarnieri F, Longo DL, Kwak LW (1996) Immunization with granulocyte-macrophage colony-stimulating factor-transduced, but not B7-1-transduced, lymphoma cells primes idiotype-specific T cells and generates potent systemic antitumor immunity. J Immunol 156:3858–3865
Meziane el K, Bhattacharyya T, Armstrong AC, Qian C, Hawkins RE, Stern PL, Dermime S (2004) Use of adenoviruses encoding CD40L or IL-2 against B cell lymphoma. Int J Cancer 111:910–920
Siegel S, Wagner A, Kabelitz D, Marget M, Coggin J Jr, Barsoum A, Rohrer J, Schmitz N, Zeis M (2003) Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood 102:4416–4423
Siegel S, Wagner A, Schmitz N, Zeis M (2003) Induction of antitumour immunity using survivin peptide-pulsed dendritic cells in a murine lymphoma model. Br J Haematol 122:911–914
Zibert A, Balzer S, Souquet M, Quang TH, Paris-Scholz C, Roskrow M, Dilloo D (2004) CCL3/MIP-1alpha is a potent immunostimulator when coexpressed with interleukin-2 or granulocyte-macrophage colony-stimulating factor in a leukemia/lymphoma vaccine. Hum Gene Ther 15:21–34
Rieger R, Kipps TJ (2003) CpG oligodeoxynucleotides enhance the capacity of adenovirus-mediated CD154 gene transfer to generate effective B-cell lymphoma vaccines. Cancer Res 63:4128–4135
Curti A, Parenza M, Colombo MP (2003) Autologous and MHC class I-negative allogeneic tumor cells secreting IL-12 together cure disseminated A20 lymphoma. Blood 101:568–575
Passineau MJ, Siegal GP, Everts M, Pereboev A, Jhala D, Wang M, Zhu ZB, Park SK, Curiel DT, Nelson GM (2005) The natural history of a novel, systemic, disseminated model of syngeneic mouse B-cell lymphoma. Leuk Lymphoma 46:1627–1638
Molinier-Frenkel V, Lengagne R, Gaden F, Hong SS, Choppin J, Gahery-Segard H, Boulanger P, Guillet JG (2002) Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J Virol 76:127–135
Graham MM, Peterson LM, Hayward RM (2000) Comparison of simplified quantitative analyses of FDG uptake. Nucl Med Biol 27:647–655
Lee JR, Madsen MT, Bushnel D, Menda Y (2000) A threshold method to improve standardized uptake value reproducibility. Nucl Med Commun 21:685–690
Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R (1979) Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol 122:549–554
Lotze MT, Papamichail M (2004) A primer on cancer immunology and immunotherapy. Cancer Immunol Immunother 53:135–138
Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH (2003) Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med 198:1023–1034
Hoffman EJ, Huang SC, Phelps ME (1979) Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr 3:299–308
Israel O, Yefremov N, Bar-Shalom R, Kagana O, Frenkel A, Keidar Z, Fischer D (2005) PET/CT detection of unexpected gastrointestinal foci of 18F-FDG uptake: incidence, localization patterns, and clinical significance. J Nucl Med 46:758–762
Spaepen K, Stroobants S, Dupont P, Bormans G, Balzarini J, Verhoef G, Mortelmans L, Vandenberghe P, De Wolf-Peeters C (2003) [(18)F]FDG PET monitoring of tumour response to chemotherapy: does [(18)F]FDG uptake correlate with the viable tumour cell fraction? Eur J Nucl Med Mol Imaging 30:682–688
Yamada K, Brink I, Bisse E, Epting T, Engelhardt R (2005) Factors influencing [F-18] 2-fluoro-2-deoxy-d-glucose (F-18 FDG) uptake in melanoma cells: the role of proliferation rate, viability, glucose transporter expression and hexokinase activity. J Dermatol 32:316–334
Uhr JW, Marches R (2001) Dormancy in a model of murine B cell lymphoma. Semin Cancer Biol 11:277–283
Spaepen K, Stroobants S, Dupont P, Van Steenweghen S, Thomas J, Vandenberghe P, Vanuytsel L, Bormans G, Balzarini J, De Wolf-Peeters C, Mortelmans L, Verhoef G (2001) Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol 19:414–419
Buchmann I, Reinhardt M, Elsner K, Bunjes D, Altehoefer C, Finke J, Moser E, Glatting G, Kotzerke J, Guhlmann CA, Schirrmeister H, Reske SN (2001) 2-(fluorine-18)fluoro-2-deoxy-d-glucose positron emission tomography in the detection and staging of malignant lymphoma. A bicenter trial Cancer 91:889–899
Bankert RB, Egilmez NK, Hess SD (2001) Human-SCID mouse chimeric models for the evaluation of anti-cancer therapies. Trends Immunol 22:386–393
Acknowledgments
This work was supported by the French Association for Cancer Research (ARC grant no. 3257) and by ARTGIL (granted by Amgen and Roche France). C. Chaise was initially supported by a grant from the French ministry for education and research and subsequently from the Fondation pour la Recherche Médicale (FRM). We thank Gaël Grannec and Sandrine Machane for their help with animal care, Dr Fabrice Chrétien for photographs of the animals and Dr William Hempel for careful editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Chaise and E. Itti contributed equally to the work.
Rights and permissions
About this article
Cite this article
Chaise, C., Itti, E., Petegnief, Y. et al. [F-18]-Fluoro-2-deoxy-d-glucose positron emission tomography as a tool for early detection of immunotherapy response in a murine B cell lymphoma model. Cancer Immunol Immunother 56, 1163–1171 (2007). https://doi.org/10.1007/s00262-006-0265-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-006-0265-0