Abstract
Purpose
To assess whether the application of a preparatory micro-enema reduces gas-induced susceptibility artefacts on diffusion-weighted MRI of the prostate.
Methods
114 consecutive patients who received multiparametric 3 T MRI of the prostate at our institution were retrospectively enrolled. 63 patients self-administered a preparatory micro-enema prior to imaging, and 51 patients underwent MRI without bowel preparation. Two blinded readers independently reviewed the diffusion-weighted sequences regarding gas-induced artefacts. The presence/severity of artefacts was scored ranging from 0 (no artefact) to 3 (severe artefact). A score ≥ 2 was considered a clinically relevant artefact. Maximum rectal width at the level of the prostate was correlated with the administration of a micro-enema. Scores were compared between the scans performed with and without bowel preparation using univariable and multivariable logistic regression, taking into account potential confounding factors (age and prostate volume).
Results
Significantly less artefacts were found on diffusion-weighted sequences after the administration of a micro-enema shortly prior to MR imaging. Clinically relevant artefacts were found in 10% in the patient group after enema, in 41% without enema. If present, artefacts were also significantly less severe. Mean severity score was 0.3 (enema administered) and 1.2 (no enema), and odds ratio was 0.137 (p < 0.0001) in univariable ordinal logistic regression. Inter-observer agreement was excellent (κ 0.801).
Conclusion
The use of a preparatory micro-enema prior to 3 T multiparametric prostate MRI significantly reduces both the incidence and severity of gas-induced artefacts on diffusion-weighted sequences and thus improves image quality.
Similar content being viewed by others
Introduction
Prostate cancer is the second most common cancer worldwide and the fifth most common cause of death due to cancer among men, with a cumulative life risk of 3.73% [1]. The standard diagnostic work-up for prostate cancer is based on clinical examination including measurement of serum prostate-specific antigen (PSA) and digital rectal examination (DRE) [2]. Multiparametric magnetic resonance imaging (mpMRI) of the prostate combines anatomical and functional imaging and has become increasingly important in the diagnosis and treatment planning of prostate cancer. Its use in various settings has been discussed and recommended in several prostate cancer guidelines worldwide [2,3,4,5]. The Prostate Imaging Reporting and Data System, PIRADS v2.1, requires mpMRI, including high-resolution T2-weighted, diffusion-weighted and dynamic contrast-enhanced T1-weighted sequences at field strengths of at least 1.5 T [6].
High-resolution T2-weighted sequences have long been the mainstay of prostate imaging, but there is convincing evidence that the addition of diffusion-weighted imaging (DWI) leads to greatly improved diagnostic performance [7,8,9]. DWI is now an integral part of prostate MRI and shows promising results as a possible tool for risk stratification. Several studies have postulated that DWI might be useful to distinguish between low-risk lesions, which could be managed by active surveillance, and high-risk lesions, requiring a more aggressive therapeutic approach [10,11,12,13,14,15]. Moreover, DWI could also be useful as a tool to assess treatment response, e.g. to androgen-deprivation therapy, targeted photodynamic therapy or radiotherapy, as well as in the detection of recurrence [16,17,18,19,20,21]. Dynamic contrast-enhanced T1-weighted sequences, however, have recently been described as less essential for MRI of the prostate, and shorter, biparametric protocols consisting of T2-weighted and diffusion-weighted sequences have been proposed [22,23,24,25,26,27].
DWI, especially commonly used single-shot echo-planar sequences, is prone to susceptibility-related artefacts which occur at the interfaces of structures with different magnetic susceptibilities, such as air and soft tissue. These artefacts, which cause magnetic field inhomogeneities with image distortion and/or signal dropout, tend to be more pronounced at 3 T compared to lower field strengths [28,29,30]. During mpMRI of the prostate, air in the rectum may significantly reduce image quality of DWI due to pronounced distortion artefacts [31, 32].
Preparatory enemas to overcome bowel gas-related susceptibility artefacts on DWI of the rectum and prostate have been discussed in the literature. However, published data are conflicting, with some publications stating no benefit, others claiming improved image quality after the administration of a micro-enema [33,34,35]. As a result, PIRADS v2.1 discusses the implementation of bowel preparation, stating that, so far, no consensus regarding patient preparation has been reached. A preparatory enema might be helpful, but there are concerns regarding increased bowel motility and consequent motion artefacts [6].
Thus, the aim of our study was to assess the use of a preparatory micro-enema prior to multiparametric prostate MRI.
Material and methods
Patients
The study was approved by the local ethics committee. Due to the retrospective nature of this study, informed consent was waived.
MRIs and clinical data of 126 consecutive patients who had received mpMRI of the prostate at our institution between January 2017 and July 2017 were retrospectively reviewed. In March 2017, we had implemented a new protocol for mpMRI of the prostate including bowel preparation. This was done in the hopes to reduce the occurrence of susceptibility artefacts on DWI and after hearing promising results in regards to bowel preparation at 3 T MRI of the rectum [36].
To avoid bias, all repeat MRIs of the same patient were excluded. Eight patients were excluded because of artefacts due to hip replacements. One patient after rectal resection, one with pronounced general motion artefacts and two patients with indwelling urinary catheters were also excluded. The remaining 114 patients were included in the study. 63 patients self-administered a preparatory micro-enema prior to imaging, 51 patients underwent MRI without bowel preparation, either because they were examined prior to the implementation of the bowel preparation protocol or because they did not consent to the application. We did not perform standardized interviews after the MRI, but patients generally tolerated the micro-enema well. Patient characteristics and indications for MRI are summarized in Tables 1 and 2.
MR imaging
Patients were asked to self-administer a micro-enema (Microlax®, 5 ml, Johnson & Johnson) 15–30 min prior to MRI. No dietary instructions were given to patients and no other bowel preparation or spasmolytics were administered. PIRADS v2.1 discusses the administration of spasmolytics, but there is no recommendation as to routine use due to the unclear benefit, incremental cost and potential side effects [6].
All MR images were acquired on a 3 T scanner (Siemens Verio, Siemens AG, Medical Solutions) using a phased-array surface coil and a standardized routine protocol for prostate imaging. The protocol consists of one axial T1-weighted sequence, two T2-weighted sequences (sagittal and axial), axial diffusion-weighted sequences (five b values), axial dynamic contrast-enhanced T1-weighted sequences after administration of 0.1 ml/kg Gadobutrol (Gadovist®, Bayer Vital GmbH) intravenously, including delayed contrast-enhanced, fat-saturated T1-weighted images about 5 min post contrast injection. Apparent diffusion coefficient maps were generated automatically. For detailed sequence parameters of the diffusion-weighted sequences see Table 3.
Image interpretation
MR images were reviewed at a PACS workstation (AGFA Healthcare, Impax EE R20 XVIII SU1). Two readers (one radiologist with more than 7 years, one radiology resident with more than 2 years of experience in multiparametric prostate MRI) independently reviewed DWI b1000-images of all cases for bowel-gas-related susceptibility artefacts with a negative impact on the evaluation of the prostate. Both readers also reviewed T2-weighted sequences for motion artefacts and other exclusion criteria (hip replacement, rectal resection, indwelling urinary catheter). The readers were blinded to the date of the MRI, medical history, bowel preparation and referral diagnoses.
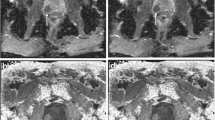
Artefacts were scored from 0 to 3 (0 = no artefacts, 1 = mild artefacts, 2 = moderate artefacts, 3 = severe artefacts). No artefact was defined as good delineation of prostate contours and no image distortion of the prostate, mild artefact as mild distortion, with the bigger part of the prostate still amenable to imaging analysis. Moderate artefact was defined as marked distortion and/or blur concerning the bigger part of the prostate, with some remaining areas still assessable, severe artefact as severe distortion with severe signal pile-up, leading to non-diagnostic images (see Figs. 1, 2, 3, 4). Prior to reviewing patient scans, both readers were trained using exemplary cases to ensure consistent artefact scoring. The maximum width (measured on the sagittal T2-weighted sequence) of the rectum at the level of the prostate was also documented. Prostate volume was calculated using the following formula: Length × Width × Height × π/6. Prostate volume and maximum rectal width were determined by both readers in consensus.
Analysis of clinical data
Patient files were reviewed to determine patient age and indication for MRI.
Statistical analysis
We used descriptive statistics and the Shapiro–Wilk test to assess the normal distribution for numerical variables. As the data were not normally distributed, we used the Mann–Whitney U test to assess differences between both groups (enema versus no-enema group). For the purpose of statistical analysis, artefact scores of ≤ 1 (no/mild artefacts) and ≥ 2 (moderate/severe artefacts) were pooled and an artefact score ≥ 2 was defined as clinically relevant artefacts. The proportion of clinically relevant artefacts in the no micro-enema versus micro-enema group was assessed as a primary outcome, the artefact severity score itself as a secondary outcome. Univariate analysis using Mann–Whitney U test was performed to assess the differences between both groups regarding rectal width. Univariable and multivariable logistic regression was used to assess potential confounding factors (age and prostate volume), with a binary outcome for the primary outcome and ordinal logistic regression for the secondary outcome. p < 0.05 was considered to indicate statistically significant differences. Inter-observer agreement was assessed using Cohen’s Kappa (0–0.2 = poor, 0.21–0.4 = fair, 0.41–0.6 = moderate, 0.61–0.8 = good and 0.81–1.00 = excellent agreement).
Statistical analysis was performed using SPSS package for Windows, SPSS Statistics 23, IBM.
Results
Mean age of all patients was 65 years (30–83 years). Mean prostate volume measured 58.3 ml (9.5–249.3 ml). Age and prostate volume were not statistically different between both groups of patients (no enema/enema, p ≥ 0.05). In both groups, there were two patients after TURP.
Diffusion-weighted sequences on MRI of patients in the group after self-administration of micro-enema showed significantly less clinically relevant (≥ 2) artefacts compared to the no-enema group, with an odds ratio of 0.150 (p = 0.0002). Clinically significant artefacts were present in 10% in the enema group and in 41% in the no-enema group. Artefacts were also significantly less severe in the enema group, with an odds ratio of 0.137 (p < 0.0001). The mean severity score in the enema group was 0.3 versus 1.2 in the no-enema group (see Table 4, Fig. 5). Rectal width significantly reduced after enema in univariate analysis. Maximum width of the rectal ampulla was 2.56 cm in the enema group, compared to 3.19 cm in the no-enema group.
Inter-observer agreement was excellent (κ 0.801, 95% confidence interval 0.69–0.88).
Discussion
Our study demonstrates that bowel preparation using a self-administered micro-enema is feasible and leads to significantly fewer, as well as less severe artefacts on DWI sequences of the prostate. The additional cost of bowel preparation in this setting is low (ca. 1 € per micro-enema) and, although we did not perform standardized interviews, patients generally tolerated the micro-enema well.
Prior studies have reported conflicting results, with one study by Lim et al. finding no significant reduction of artefacts or improvement of image quality after the use of a preparatory enema prior to mpMRI of the prostate at 3 T [33]. This retrospective study included 60 patients who were asked to administer a cleansing enema (Fleet enema, C.B. Fleet) the morning of the scheduled MRI. Several factors might have led to the results, which are contrary to our findings. The authors do not provide data on the time lag between administration of the enema and MRI, but relatively long delays may have had a negative impact on artefact reduction. Rectal stool and gas in the study by Lim et al. were both scored on a five-point scale (no rectal stool/gas to large amount of rectal stool/gas), based on subjective assessment, which could introduce bias into the findings. In our study, we also subjectively assessed and scored artefacts, but included measurements of the maximum rectal width at the level of the prostate. Maximum rectal width, depending on the amount of stool/air in the rectal ampulla, was significantly lower after the administration of a micro-enema (p < 0.001). Only 16% of patients in the no-enema group were reported by Lim et al. as showing moderate to large amounts of stool and no patient in either group was recorded as having moderate to large amounts of rectal gas, which could explain why a preparatory enema did not lead to a significant reduction in artefacts. Also, the number of patients included was relatively low (with 28 patients having received bowel preparation).
Another recent study retrospectively evaluated 117 patients under active surveillance who underwent prostate MRI both without and with bowel preparation obtained within 12 months of each other [34]. In this study, fleet’s enema was administered about 12 h prior to the MRI scan. Two readers (one radiologist, one urologist) reviewed images regarding rectal distension, rectal anterior–posterior (AP) diameter, DWI distortion and DWI artefacts. DWI distortion and rectal AP diameter were assessed quantitatively, whereas rectal distension and DWI artefacts were assessed using scoring systems. The results are partially in keeping with our findings, insofar as rectal diameter was significantly lower in the patient group with bowel preparation. The radiologist reader also found rectal distension on a subjective Likert scale to be significantly lower in this group, whereas for the urologist reader this was not significant.
Regarding DWI assessment, only the radiologist reader found significant improvement of DWI distortion after bowel preparation, both readers did not find significant differences in DWI artefacts on qualitative scoring. Inter-observer agreement in this study by Coskun et al. was weak for DWI distortion and artefacts. A possible explanation could be that the urologist reader approached imaging and especially artefact assessment from a different point of view, presumably being primarily trained to look for malignancy. Artefact scoring was performed reviewing scans for blurring or subtle artefact lines, poor signal-to-noise ratio or prominent artefact lines, but no training was performed prior to the evaluation and no image examples demonstrating artefact scoring are provided. We tried to standardize subjective artefact scoring by training readers as a first step and by employing specific definitions of artefact scores, which led to excellent inter-observer agreement. Another interesting point is that mean rectal AP diameter without bowel preparation was markedly lower than in our study (2.67 versus 3.19 cm), whereas mean diameter after enema was similar (2.48 versus 2.56 cm). Bowel preparation is more useful, the more stool/gas is found in the rectal ampulla, which could be another explanation for the difference in results and conclusion. Lastly, including patients receiving mpMRI within a 12-month period might have led to significant changes like progressive benign prostate hyperplasia or intercurrent repeat biopsy.
A retrospective study by Griethuysen et al. included 335 MRI scans of the rectum at 1.5 T. Patients were asked to self-administer a cleansing enema shortly prior to MRI. The authors concluded that the incidence and severity of gas-induced artefacts on DWI was significantly reduced after the administration of a micro-enema [35]. Although this study examined patients who received MRI of the rectum, methodology was similar and the results support our findings.
Several MRI techniques have been reported to reduce artefacts in diffusion-weighted imaging, such as reduction of the field-of-view, the application of segmented read-out epi sequences or parallel imaging [37,38,39,40]. However, Griethuysen et al. concluded that the effect of these techniques was less than that of a preparatory micro-enema (combined with parallel imaging) [35].
Interestingly, we did not encounter an increased rate of motion artefacts after the administration of the micro-enema, which has been a concern described in the literature [6]. We did not administer spasmolytics, but had to exclude only one patient due to pronounced motion artefacts. This could be due to the fact that we used only a micro-enema (5 ml), instead of a larger enema, which might lead to more pronounced bowel motion.
There are several limitations to our study. Firstly, data were analysed retrospectively and we did not perform test–retest evaluations. Secondly, the score implemented to quantify the presence and severity of artefacts is based on subjective visual assessment, which might hamper reproducibility. We tried to counter this limitation by using a standardized scoring system, for which readers received a short training course prior to reviewing the MRI scans. As a result, excellent inter-observer agreement between the two readers was reached. Thirdly, readers were blinded to the administration of micro-enema but suggestive imaging findings such as fluid, but no stool and/or gas in the rectal ampulla might have introduced a bias as to patient preparation. Another limitation is the inter-individual study design, although patient characteristics were comparable in both groups. Since our aim was to evaluate the influence of a micro-enema on time-efficient diffusion-weighted imaging in a clinical routine setting, we kept all other imaging parameters constant. Combining the administration of a micro-enema with different imaging techniques like non-epi read-out, different excitation methods or B0 mapping could lead to further reduction of artefacts.
In conclusion, our study provides evidence that a preparatory micro-enema prior to mpMRI of the prostate is feasible and leads to improved image quality on DWI. Prospective studies are necessary to confirm our results, and the combination of advanced imaging techniques with bowel preparation should be explored further.
Data availability
All data and material are available from the corresponding author.
Change history
23 June 2021
The following OA funding note is addressed above reference section: Open Access funding enabled and organized by Projekt DEAL.
06 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00261-021-03157-x
Abbreviations
- PSA:
-
Prostate specific antigen
- DRE:
-
Digital rectal examination
- TRUS:
-
Transrectal ultrasound
- mpMRI:
-
Multiparametric MRI
- EPI:
-
Echo planar imaging
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J Clin 68:394-424
Barentsz JO, Richenberg J, Clements R, et al. (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22(4):746–757
Carrol PR, Kellogg Parsons J, Andriole G, et al. (2016) NCCN Guidelines Insights Prostate Cancer Early Detection, Version 2.2016 Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw 14(5):509–519
National Institute for Health and Care Excellence (2019) Prostate cancer: diagnosis and management. National Institute for Health and Care Excellence. Available via https://www.nice.org.uk/guidance/ng131/resources/prostate-cancer-diagnosis-and-management-pdf-66141714312133. Accessed 28 August 2019
Richenberg J, Løgager V, Panebianco V, Rouviere O, Villeirs G, Schoots IG (2019) The primacy of multiparametric MRI in men with suspected prostate cancer. Eur Radiol Dec;29(12):6940–6952
Turkbey B, Rosenkrantz AB, Haider MA, et al. (2019) Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 76(3):340-351
Tan CH, Wei W, Johnson V, Kundra V (2012). Diffusion-weighted MRI in the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol 199(4):822–829
Jie C, Rongbo L, Ping T (2014). The value of diffusion-weighted imaging in the detection of prostate cancer: a meta-analysis. Eur Radiol 24(8):1929–1941
Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN (2012). The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol 199(1):103–110
Vargas HA, Akin O, Franiel T, et al. (2011) Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 259(3):775–784
Hambrock T, Somford DM, Huisman HJ, et al. (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–461
Bittencourt LK, Barentsz JO, de Miranda LC, Gasparetto EL (2012) Prostate MRI: diffusion-weighted imaging at 1.5T correlates better with prostatectomy Gleason Grades than TRUS-guided biopsies in peripheral zone tumours. Eur Radiol 22(2):468–475
Jung SI, Donati OF, Vargas HA, Goldman D, Hricak H, Akin O (2013) Transition zone prostate cancer: incremental value of diffusion-weighted endorectal MR imaging in tumor detection and assessment of aggressiveness. Radiology 269(2):493–503
van As NJ, de Souza NM, Riches SF, et al. (2009) A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol 56(6):981–987
Flavell RR, Westphalen AC, Liang C, et al. (2014) Abnormal findings on multiparametric prostate magnetic resonance imaging predict subsequent biopsy upgrade in patients with low risk prostate cancer managed with active surveillance. Abdom Imaging 39(5):1027–1035
Park SY, Kim CK, Park BK, Lee HM, Lee KS (2011) Prediction of biochemical recurrence following radical prostatectomy in men with prostate cancer by diffusion-weighted magnetic resonance imaging: initial results. Eur Radiol 21(5):1111–1118
Kim AY, Kim CK, Park SY, Park BK (2014) Diffusion-weighted imaging to evaluate for changes from androgen deprivation therapy in prostate cancer. AJR Am J Roentgenol 203(6):W645–650
Hotker AM, Mazaheri Y, Zheng J, et al. (2015) Prostate Cancer: assessing the effects of androgen-deprivation therapy using quantitative diffusion-weighted and dynamic contrast-enhanced MRI. Eur Radiol 29:29
Wang H, Fei B (2010) Diffusion-weighted MRI for monitoring tumor response to photodynamic therapy. J Magn Reson Imaging 32(2):409–417
Park SY, Kim CK, Park BK, et al. (2012) Early changes in apparent diffusion coefficient from diffusion-weighted MR imaging during radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 83(2):749–755
Decker G, Murtz P, Gieseke J, et al. (2014) Intensity-modulated radiotherapy of the prostate: dynamic ADC monitoring by DWI at 3.0 T. Radiother Oncol 113(1):115–120
van der Leest M, Israël B, Cornel EB et al. (2019) High Diagnostic Performance of Short Magnetic Resonance Imaging Protocols for Prostate Cancer Detection in Biopsy-naïve Men: The Next Step in Magnetic Resonance Imaging Accessibility. Eur Urol. https://doi.org/10.1016/j.eururo.2019.05.029
Obmann VC, Pahwa S, Tabayayong W, et al. (2018) Diagnostic accuracy of a rapid biparametric MRI protocol for detection of histologically proven prostate cancer. Urology 122:133–138
Sherrer RL, Glaser ZA, Gordetsky JB, Nix JW, Porter KK, Rais-Bahrami S (2018) Comparison of biparametric MRI to full multiparametric MRI for detection of clinically significant prostate cancer. Prostate Cancer Prostatic Dis. May;22(2):331–336
Boesen L, Nørgaard N, Løgager V, et al. (2018) Assessment of the diagnostic accuracy of biparametric magnetic resonance imaging for prostate cancer in biopsy-naive men: the Biparametric MRI for Detection of Prostate Cancer (BIDOC) Study. JAMA Netw Open Jun 1;1(2):e180219
Kuhl CK, Bruhn R, Kramer N, Nebelung S, Heidenreich A, Schrading S (2017) Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology 285:493–505
Niu XK, Chen XH, Chen ZF, Chen L, Li J, Peng T (2018) Diagnostic performance of biparametric MRI for detection of prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol 211:369–378
Mazaheri Y, Vargas HA, Nyman G, Akin O, Hricak H (2013) Image artifacts on prostate diffusion-weighted magnetic resonance imaging: trade-offs at 1.5 Tesla and 3.0 Tesla. Acad Radiol. 20(8):1041–1047
Kuhl CK, Gieseke J, von Falkenhausen M, et al. (2005) Sensitivity encoding for diffusion-weighted MR imaging at 3.0 T: intraindividual comparative study. Radiology 234(2):517–526
Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B (2011) Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J Magn Reson Imaging. Jan;33(1):128–135
Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T (2017) Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur. J. Radiol. 90 174–180
Rosenkrantz AB, Taneja SS (2014) Radiologist, be aware: ten pitfalls that confound the interpretation of multiparametric prostate MRI. AJR Am J Roentgenol 202:109–120
Lim C, Quon J, McInnes M, Shabana WM, El-Khodary M, Schieda N (2015) Does a cleansing enema improve image quality of 3T surface coil multiparametric prostate MRI? J Magn Reson Imaging. Sep;42(3):689–97
Coskun M, Mehralivand S, Shih JH et al. (2020) Impact of bowel preparation with Fleet’s™ enema on prostate MRI quality. Abdom Radiol Mar 25. doi: 10.1007/s00261–020–02487–6. [Epub ahead of print]
van Griethuysen JJM, Bus EM, Hauptmann M et al. (2018) Gas-induced susceptibility artefacts on diffusion-weighted MRI of the rectum at 1.5 T - Effect of applying a micro-enema to improve image quality. Eur J Radiol. 2Feb;99:131–137
van Griethuysen JJM, Bus EM, Hauptmann M et al. (2017) Air artefacts on diffusion-weighted MRI of the rectum: effect of applying a rectal micro-enema. Oral presentation at ECR 2017
Korn N, Kurhanewicz J, Banerjee S, Starobinets O, Saritas E, Noworolski S (2015) Reduced-FOV excitation decreases susceptibility artifact in diffusion-weighted MRI with endorectal coil for prostate cancer detection. Magn Reson Imaging. Jan;33(1):56–62
Thian YL, Xie W, Porter DA, Weileng Ang B (2014) Readout-segmented echo-planar imaging for diffusion-weighted imaging in the pelvis at 3T-A feasibility study. Acad Radiol. Apr;21(4):531–537
Li L, Wang L, Deng M et al. (2015) Feasibility Study of 3-T DWI of the Prostate: Readout-Segmented Versus Single-Shot Echo-Planar Imaging AJR Am J Roentgenol. Jul;205(1):70–76
Jambor I (2017) Optimization of prostate MRI acquisition and post-processing protocol: a pictorial review with access to acquisition protocols. Acta Radiol Open Dec 8;6(12):2058460117745574
Acknowledgements
Preiss-Daimler Stiftung Medical Equipment and Research.
Funding
The corresponding author received a research grant from the Preiss-Daimler Stiftung Medical Equipment and Research. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
VP: Corresponding author; CGR: Substantial contribution to the design, analysis and interpretation of the data; H-MH: Substantial contribution to the design, acquisition and interpretation of the data; CB: Substantial contribution to the design, acquisition and interpretation of the data. AB: Substantial contribution to the analysis and interpretation of the data as well as critical revision. CT: Substantial contribution to the analysis and interpretation of the data as well as critical revision; J-PK: Substantial contribution to the analysis and interpretation of the data as well as critical revision; ML: Substantial contribution to the analysis and interpretation of the data as well as critical revision; R-TH: Substantial contribution to the analysis and interpretation of the data as well as critical revision; IP: Substantial contribution to the analysis and interpretation of the data as well as critical revision. All authors reviewed and approved the work to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The corresponding author owns 5 Siemens shares (not Siemens Healthineers).
Ethics approval
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval of the local ethics committee at the Technical University Dresden was granted prior to the study.
Consent to participate/publish
Due to the retrospective nature of the study informed consent was waived by the local ethics committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised due to a retrospective open access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Plodeck, V., Radosa, C.G., Hübner, HM. et al. Rectal gas-induced susceptibility artefacts on prostate diffusion-weighted MRI with epi read-out at 3.0 T: does a preparatory micro-enema improve image quality?. Abdom Radiol 45, 4244–4251 (2020). https://doi.org/10.1007/s00261-020-02600-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02600-9