Abstract
Purpose
The purpose of the study was to determine if enhancement features and qualitative imaging features on multiphasic multidetector computed tomography (MDCT) were associated with tumor grade in patients with clear cell renal cell carcinoma (ccRCC).
Methods
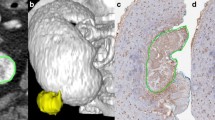
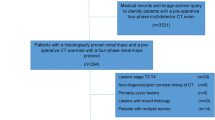
In this retrospective, IRB approved, HIPAA-compliant, institutional review board-approved study with waiver of informed consent, 127 consecutive patients with 89 low grade (LG) and 43 high grade (HG) ccRCCs underwent preoperative four-phase MDCT in unenhanced (UN), corticomedullary (CM), nephrographic (NP), and excretory (EX) phases. Previously published quantitative (absolute peak lesion enhancement, absolute peak lesion enhancement relative to normal enhancing renal cortex, 3D whole lesion enhancement and the wash-in/wash-out of enhancement within the 3D whole lesion ROI) and qualitative (enhancement pattern; presence of necrosis; pattern of; tumor margin; tumor–parenchymal interface, tumor–parenchymal interaction; intratumoral vascularity; collecting system infiltration; renal vein invasion; and calcification) assessments were obtained for each lesion independently by two fellowship-trained genitourinary radiologists. Comparisons between variables included χ2, ANOVA, and student t test. p values less than 0.05 were considered to be significant. Inter-reader agreement was obtained with the Gwet agreement coefficient (AC1) and standard error (SE) was reported.
Results
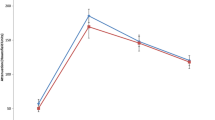
No significant differences were observed between the LG and HG ccRCC cohorts with respect to absolute peak lesion enhancement and relative lesion enhancement ratio. There was a significant inverse correlation between low and high grade ccRCC and tumor enhancement the NP (71 HU vs. 54 HU, p < 0.001) and EX (52 HU vs. 39 HU, p < 0.001) phases using the 3D whole lesion ROI method. The percent wash-in of 3D enhancement from the UN to the CM phase was also significantly different between LG and HG ccRCCs (352% vs. 255%, p = 0.003). HG lesions showed significantly more calcification, necrosis, collecting system infiltration and ill-defined tumor margins (p < 0.05). Overall agreement between the two readers had a mean AC1 of 0.8172 (SE 0.0235).
Conclusions
Quantitatively, high grade ccRCC had significantly lower whole lesion enhancement in the NP and EX phases on MDCT. Qualitatively, high grade ccRCC were significantly more likely to be associated with calcifications, necrosis, collecting system infiltration, and an ill-defined tumor margin.







Similar content being viewed by others
References
Global Burden of Disease Cancer Collaboration (2015) The global burden of cancer 2013. JAMA Oncol 1:505–527
Lam JS, Shvarts O, Leppart JT, et al. (2005) Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol 174:466–472
Sun M, Shariat SF, Cheng C, et al. (2011) Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 60(4):644–661
Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6(7):655–663
Delahunt B, Egevad L, Montironi R, et al. (2013) International Society of Urological Pathology (ISUP) consensus conference on renal neoplasia: rationale and organization. Am J Surg Pathol 37:1463–1468
Silverman SG, Israel GM, Trinh QD (2015) Incompletely characterized incidental renal masses: emerging data support conservative management. Radiology 275(1):28–42
Lebret T, Poulain JE, Molinie V, et al. (2007) Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol 178(4 Pt 1):1184–1188 (discussion 1188. 75)
Leveridge MJ, Finelli A, Kachura JR, et al. (2011) Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 60(3):578–584
Halverson SJ, Kunju LP, Bhalla R, et al. (2013) Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol 189(2):441–446
Ball MW, Bezerra SM, Gorin MA, et al. (2015) Grade heterogeneity in small renal masses: potential implications for renal mass biopsy. J Urol 193(1):36–40
Gerlinger Rowan, Horswell S, et al. (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892
Jamshidi N, Jonasch E, Zapala M, et al. (2015) The radiogenomic risk score: construction of a prognostic quantitative, noninvasive image-based molecular assay for renal cell carcinoma. Radiology 277:114–123
Lubner M, Stabo N, Abel EJ, Munoz del Rio A, Pickhardt PCT (2016) Textural analysis of large primary renal cell carcinomas: pretreatment tumor heterogeneity correlates with histologic findings and clinical outcomes. AJR 207:96–105
Yin Q, Hung SC, Wang L, et al. (2017) Associations between tumor vascularity, vascular endothelial growth factor expression and PET/MRI radiomic signatures in primary clear cell renal cell carcinoma: proof of concept study. Sci Rep 7:43356. https://doi.org/10.1038/srep43356
Shinagare A, Krajewski K, Braschi-Amirfarzan M, Ramaiya N (2017) Advanced renal cell carcinoma: role of the radiologist in the era of precision medicine. Radiology 284(2):333–351
Ishigami K, Leite L, Pakalniskis M, et al. (2014) Tumor grade of clear cell renal cell carcinoma assessed by contrast-enhanced computed tomomgraphy. SpringerPlus 3:694
Rosenkrantz AB, Niver BE, Fitzgerald EF, et al. (2010) Utility of the apparent diffusion coefficient for distinguishing clear cell renal cell carcinoma of low and high nuclear grade. Am J Roentgenol 195:W344–W351
Reiner C, Roessle M, Thiesler T, et al. (2013) Computed tomography perfusion imaging of renal cell carcinoma. Invest Radiol 48:183–191
Jinzaki M, Tanimoto A, Mukai M, et al. (2000) Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr 24(6):835–842
Villalobos-Gollas M, Aguilar-Davidov B, Culebro-Garcia C, et al. (2012) Pathological implications of areas of lower enhancement on contrast-enhanced computed tomography in renal cell carcinoma: additional information for selecting candidates for surveillance protocols. Int Urol Nephrol 44:1369–1374
Yuan Q, Kapur P, Zhang Y (2016) Intratumor heterogeneity of perfusion and diffusion in clear-cell renal cell carcinoma: correlation with tumor cellularity. Clin Genitourin Cancer 14(6):e585–e594
Vargas H, Delany H, Delappe E, et al. (2013) Multiphasic contrast-enhanced MRI: single-slice versus volumetric quantification of tumor enhancement for assessment of renal clear-cell carcinoma fuhrman grade. J Magn Reson Imaging 37:1160–1167
Frank I, Blute ML, Cheville JC, et al. (2002) An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 168(6):2395–2400
Leibovich BC, Blute ML, Cheville JC, et al. (2003) Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 97(7):1663–1671
Sahni VA, Silverman SG (2014) Imaging management of incidentally detected small renal masses. Semin Interv Radiol 31(1):9–19
Coy H, Young JR, Douek M, et al. (2017) Quantitative computer-aided diagnostic algorithm for automated detection of peak lesion attenuation in differentiating clear cell from papillary and chromophobe renal cell carcinoma, oncocytoma, and fat-poor angiomyolipoma on multiphasic multidetector computed tomography. Abdom Radiol 42(7):1919–1928
Zhu YH, Wang X, Zhang J, et al. (2014) Low enhancement on multiphase contrast-enhanced CT images: an independent predictor of the presence of high grade tumor of clear cell renal cell carcinoma. Am J Roentgenol 203:W295–W300
Huhdanpaa H, Hwang D, Cen S, et al. (2015) CT prediction of the Fuhrman grade of clear cell renal cell carcinoma (RCC): towards the development of computer-assisted diagnostic method. Abdom Imaging 40(8):3168–3174
Kopp RP, Aganovic L, Palazzi KL, et al. (2013) Differentiation of clear from non-clear cell renal cell carcinoma using CT washout formula. Can J Urol 20(3):6790–6797
Sun M, Lughezani G, Jeldres C, et al. (2009) A proposal for reclassification of the Fuhrman grading system in patients with clear cell renal cell carcinoma. Eur Urol 56:775–781
Oh S, Sung DJ, Yang KS, et al. (2017) Correlation of CT imaging features and tumor size with Fuhrman grade of clear cell renal cell carcinoma. Acta Radiol 58(3):376–384
Frank I, Blute ML, Cheville JC, et al. (2002) An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 168:2395–2400
Vargas H, Delany H, Delappe E, et al. (2013) Multiphasic contrast-enhanced MRI: single-slice versus volumetric quantification of tumor enhancement for assessment of renal clear-cell carcinoma Fuhrman grade. J Magn Reson Imaging 37:1160–1167
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Informed consent
This retrospective, single-center, Health Insurance Portability and Accountability Act-compliant study was approved by the Institutional Review board and a waiver of informed consent was obtained.
Rights and permissions
About this article
Cite this article
Coy, H., Young, J.R., Douek, M.L. et al. Association of qualitative and quantitative imaging features on multiphasic multidetector CT with tumor grade in clear cell renal cell carcinoma. Abdom Radiol 44, 180–189 (2019). https://doi.org/10.1007/s00261-018-1688-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1688-8