Abstract
The introduction of ultrasound contrast agents has rendered contrast-enhanced ultrasound (CEUS) a valuable complementary technique to address clinically significant problems. This pictorial review describes the use of CEUS guidance in abdominal intervention and illustrates such application for a range of clinical indications. Clinical application of CEUS discussed include commonly performed abdominal interventional procedures, such as biopsy, drainage, nephrostomy, biliary intervention, abdominal tumor ablation and its subsequent monitoring, and imaging of vascular complications following abdominal intervention. The purpose of this article is to further familiarize readers with the application of CEUS, particularly its specific strength over alternative imaging modalities, in abdominal intervention.
Similar content being viewed by others
Image guidance improves the safety and effectiveness of abdominal intervention and creates opportunities for minimal invasive therapies [1,2,3,4,5]. Ultrasound (US), fluoroscopy, and computed tomography (CT) are commonly used for image-guided procedures. US confers many distinct benefits [6]: dynamic multiplanar imaging with continuous real-time visualization of needle placement and target, repeatability with absence of ionizing radiation, good patient tolerability, and portability with the potential to be performed at any location in a variety of clinical settings. However, the limitation of lack of enhancement information with conventional US, in comparison with other enhanced imaging modalities such as CT, remains a major technical factor which hinders success of US-guided procedures [7]. Targets such as abdominal masses and complex intra-abdominal collections may be isoechoic to the background and, therefore, being poorly differentiated from adjacent structures. Procedures initially considered for US guidance may ultimately be performed with CT guidance due to the difficulty with target visualization [8]. When a procedure initially attempted with US is unsuccessful and requires an alternative imaging modality, it can result in higher costs, increased patient anxiety and discomfort, and potentially prolonged procedure time and logistical scheduling conflicts [9].
With the introduction of microbubble ultrasound contrast agents (UCAs), the issue of lack of enhancement with conventional US was addressed. UCA have been available in most parts of the world for more than 20 years. More recently, Lumason (Bracco Diagnostics; Monroe Township, NJ) has been granted FDA approval in the United States for abdominal applications for investigation of focal hepatic lesions in both the adult and pediatric populations [10]. There is also accumulative experience on the use of UCA for a variety of wider clinical indications including US-guided interventions, culminating in a number of published guidelines [11, 12].
Performance with contrast-enhanced ultrasound (CEUS)-guided abdominal intervention, as in other image-guided procedures, depends not only on the operator’s technical skill, but also on the knowledge embedded in the imaging technology, available tools, and existing protocols [13]. The purpose of this article is to consider the literature on CEUS-guided abdominal intervention, and to exhibit representative cases where CEUS proved valuable, to further familiarize readers with this technique. The specific strength of CEUS over conventional US and alternative imaging such as CT and fluoroscopy in abdominal intervention is also summarized in Table 1.
Ultrasound contrast agents
Microbubbles in the UCAs consist of an inert filling gas, such as sulfur hexafluoride, encapsulated by phospholipid shells [14]. Microbubbles resonate with low acoustic power and oscillate in a nonlinear fashion, and the harmonic signals produced can be selectively detected on US systems with special multipulse CEUS-specific software [15]. The biocompatible shells of microbubbles are metabolized by the liver, and filling gas exhaled by the lungs. UCA are not nephrotoxic, thus dispensing of any laboratory renal function tests prior to intravenous administration. Contraindications for the administration of UCA are few, namely, known allergic reaction to the UCA, severe pulmonary hypertension, and pregnancy [16]. Despite the low risk, resuscitation equipment should be accessible and trained personnel should be available for adverse events.
CEUS with intravascular administration of UCA
Microbubbles in UCA remain exclusively intravascular when administered intravenously, making them ideal for assessment of both micro- and macro vasculatures. Visualization of the vascularity of an abnormality offered by CEUS conveys its use to image-guided intervention through improved localization of a focal abnormality. Conversely, lack of perfusion can be established conclusively in avascular abnormalities such as abscesses.
CEUS should be performed after an unenhanced conventional US examination is fully evaluated. This allows the operator to identify the area of interest, and establish suitability of a subsequent CEUS examination. UCA are most commonly manually injected as a bolus through an intravenous line, usually via an antecubital vein, followed by 10 mL of 0.9% normal saline flush. It is generally best to insert the intravenous line after the baseline conventional US examination to avoid unnecessary cannulation in case a CEUS examination is not deemed worthwhile. However, due to logistical reasons, patients who are likely to benefit from intravenous UCA administration may need placement of an intravenous line prior to conventional US study at many facilities. The volume of UCA administered varies depending on the structure investigated, sensitivity of ultrasound machines and the UCA used. Conventionally, intravenous use of UCA requires 1.2–4.8 mL depending on the site examined. A dose of 2.4 mL of Lumason per injection is considered adequate for the liver and other abdominal procedures. After administration by intravenous injection, UCA lasts for about 4–5 min in the circulation. A second dose of equal volume can be administered if required.
CEUS with endocavitary administration of UCA
Endocavitary CEUS is a developing technique [17, 18]. Manual injection of air through agitated saline solution has been utilized in many endocavitary ultrasound applications [19, 20] as the technique is inexpensive and readily available. Ultrasonography contrast media for endocavitary application has, however, evolved with new microbubble UCA with improved contrast media stabilization and small but sufficiently echogenic microbubbles, resulting in superior image quality. It is currently recommended that endocavitary CEUS could be considered if alternative imaging methods carry a higher risk for the patient, e.g., need to transport critically ill patients [11]. Broadly speaking, clinical indications for endocavitary CEUS include confirmation of drain placement and effective drainage, evaluation of a physiological or non-physiological cavity, and detection of communication between two cavities through a fistula tract. The use of endocavitary CEUS in the biliary system [21], urinary collecting system [22], and “hysterosalpingo–contrast sonography” [23] has been evaluated in clinical studies.
Endocavitary CEUS differs from intravenous CEUS, in that the volume of the solvent is much smaller than the full blood circulation volume, so a much smaller dose of UCA is required. Endocavitary CEUS can be performed by first diluting UCA in 0.9% normal saline, and the resulting solution is then injected into tubes or cavities. Adequate dilution of UCA is essential to avoid posterior acoustic shadow artifacts secondary to a high concentration of microbubbles. In our experience, this could be achieved with a dilution of 0.1 mL Lumason in approximately 40–50 mL of 0.9% saline. The administered volume of the solution with diluted UCA varies on a case-by-case basis dependent on the estimated volume of the cavity. The lack of circulation of UCA in a confined cavity means microbubbles remains stable within a collection for up to 20–30 min [24]. Destruction of microbubbles by a high-energy ultrasound pulse is, therefore, necessary before repeating an endocavitary CEUS evaluation. No adverse reactions relating to endocavitary CEUS have been documented in a limited number of clinical studies. Nevertheless, contraindications for intravenous administration of UCA should be taken into consideration when performing an endocavitary CEUS.
CEUS applications in abdominal intervention
Drainage
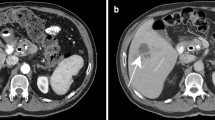
US and CT-guided percutaneous drainage is routinely used as the treatment for a variety of intra-abdominal collections. While sterile fluid collections can be easily identified on gray-scale US, complex collections such as hepatic abscesses, pancreatitis-associated fluid collections, retroperitoneal, and bowel-related abscesses, may appear almost solid and mass-like. The variation in sonographic appearances of complex intra-abdominal collections may make accurate diagnosis difficult due to the resemblance in their sonographic features to those of other pathology [25]. CEUS facilitates the identification of abscesses through demonstration of lack of vascularity within them [26]. CEUS enables better delineation of liver abscess and reveals a sharp boundary between the collection and surrounding hepatic parenchyma [27] (Fig. 1). An abscess can be more precisely targeted under CEUS guidance, with augmented depiction of the avascular portion and internal septation, allowing adequate placement of a drainage catheter. Moreover, if a collection is in close proximity to an abdominal organ, CEUS could outline the unenhanced collection precisely and minimize the risk of inadvertent injury to the adjacent organ (Fig. 2).
A Grayscale US. B CEUS of a hepatic abscess. Grayscale US image shows the abscess as a heterogeneous echogenic area with poorly defined margin underestimating the true extent of abscess. CEUS enables better delineation of the liver abscess and reveals a sharp boundary (red arrows) between the collection and hepatic parenchyma
A Grayscale US. B CEUS of a sub-capsular renal abscess. The boundary of the non-enhancing sub-capsular collection (asterisk) is clearly shown on the CEUS but not on the grayscale US image. CEUS guidance allows insertion of the drainage catheter to be performed confidently and reduces the risk of damaging the adjacent renal parenchyma
Endocavitary CEUS with injection of UCA through a drainage catheter can be performed either immediately after drainage catheter insertion or in the subsequent follow-up period. Tubes and drains are easily detected on gray-scale US but their tip positions are sometimes difficult to locate. Endocavitary CEUS through a drainage catheter primarily facilitates confirmation of the catheter position within the collections. It also provides additional assessment of effectiveness of drainage, particularly in a collection with multiple loculations [17] (Fig. 3). Furthermore, when the clinical scenario requires an answer to the question on whether any communication between a collection and an adjacent structure exists, endocavitary CEUS through a drainage tube may prove to be valuable as conventional US would be unlikely to provide sufficient information. The excellent temporal and special resolution of endocavitary CEUS allows real-time demonstration of a communication between compartments through visualization of the movement of, even a small quantity, microbubbles, without need for further alternative imaging such as contrast-enhanced fluoroscopy or CT studies (Fig. 4).
A Grayscale US. B Intravascular CEUS and C endocavitary CEUS of a hepatic abscess. Intravascular CEUS shows the enhancing hepatic parenchyma surrounding the non-enhancing abscess (red block arrow), whereas endocavitary CEUS, with the UCA instilled through the drainage tube, confirms adequate drainage catheter position and shows the morphology of the abscess cavity (asterisk)
A Intravenous CEUS. B Arterial phase image of a contrast-enhanced CT. C Endocavitary CEUS with UCA injected through the nephrostomy. D Urographic phase image of a contrast-enhanced CT of a patient with ureteric injury and urinoma formation following a vascular bypass surgery. CEUS studies (A, C) definitively excluded communication between the fluid collection and the left iliac vascular graft. Although the subsequent CT (B, D) demonstrates similar findings, CEUS provided real-time imaging and instant diagnosis at patient’s bedside
Biopsy
One of the main issue to be confronted in image-guided percutaneous biopsy procedures is the lack of imaging differentiation between the target and adjacent structures [7]. Poor differentiation of the target lesions from adjacent tissue is a common reason for failed US-guided procedures [28]. Not infrequently, a focal lesion is visible only on post-contrast diagnostic CT and cannot be adequately discerned during an US-guided biopsy procedure. CEUS is well placed to solve this problem because of its capacity to differentiate between the altered vascularization of a tumor and surrounding parenchyma [10]. CEUS guidance has been widely applied during biopsies in liver, kidneys, and other abdominal locations [29,30,31,32]. CEUS offers the potential to improve positive biopsy yield by revealing the vascularized, potentially viable or more active, portion of a lesion [33]. CEUS guidance can also be advantageous in providing spatial information of necrotic areas to avoid during biopsy, such as in biopsy of a large tumor with central necrosis [34] (Fig. 5). Furthermore, sampling of atrophic, often echogenic, renal cortex of kidneys in patients with renal insufficiency for evaluation for nephropathies [35] could be better assisted with CEUS guidance, as small, but enhancing, kidneys are better visualized on CEUS than on gray-scale US (Fig. 6).
A Grayscale US. B Color Doppler US. C Intravenous CEUS of a large renal cell carcinoma with central necrosis (red block arrow). The grayscale and color Doppler US do not show differentiation between vascular and avascular portions of the tumor. CEUS clearly shows the avascular necrotic portion (asterisk) of the tumor to be avoided during biopsy
A Grayscale US. B CEUS of an atrophic kidney targeted for a non-focal biopsy for the sampling of the renal cortex for evaluation of nephropathies. CEUS guidance improves visualization of the atrophic kidney by differentiating the enhancing renal parenchyma from the background tissue, with better delineation of the enhancing renal parenchymal outline (red arrows)
Percutaneous nephrostomy and nephrostogram
US-guided percutaneous nephrostomy (PCN) is routinely used in patients with clinical necessity of urinary drainage or urinary diversion. Frequent technical difficulties contributing to a failed PCN include lack of visibility of a target calyx [36] in cases of non-dilated collecting systems or when echogenic blood clots, pus, or debris is present within the renal calices. Intravenous CEUS improves the visibility of the calices by revealing the non-vascularized renal pelvicaliceal system against the background enhancing renal parenchyma [33]. In addition, specific techniques for CEUS-assisted puncture for PCN, with administration a small volume of diluted UCA through the puncture needle, have been described [33, 37]. With these techniques, a successful puncture can be instantly confirmed either when microbubbles reflux back along the needle due to back pressure of urine, or when microbubbles are visualized in the renal collecting system (Fig. 7). CEUS-assisted PCN offers a problem-solving adjunct in challenging cases including in non-dilated systems, showing high success rate and acceptable complications [36]. When compared to conventional fluoroscopic guidance, CEUS guidance offers a further technical advantage that if the initial placement is inaccurate, microbubbles can be destroyed and, therefore, would not leave a distracting blob of contrast material that interferes with the procedure, as in the cases of procedures performed with iodinated contrast material.
CEUS-guided nephrostomy puncture. A CEUS and B grayscale images before access into the collecting system was obtained. The stylet of the access needle for nephrostomy puncture was removed and the lumen of the needle was pre-filled with a small drop of diluted microbubble UCA. C CEUS and D grayscale US images obtained immediately after the collecting system is punctured. Microbubbles are visualized in the renal collecting system (red arrow) the instance the successful puncture is made
Following a successful PCN, it is frequently deemed necessary to assess the renal collecting system and ureter for free passage of urine prior to nephrostomy removal. The existing experience in CEUS voiding urosonography has showed safety of administration of endocavitary UCA within the urinary tract without side effects [22]. UCA can be injected through nephrostomy catheters to verify the correct placement (Fig. 8). Unobstructed drainage into the bladder can also be confirmed with a CEUS nephrostography with visualization of microbubbles in the bladder (Fig. 9). The degree of spatial resolution of endocavitary CEUS nephrostography gives a high degree of confidence of free ureteric drainage and a recent study shows 100% concordance with fluoroscopy [38]. CEUS nephrostography thus has the potential to become a feasible alternative to conventional fluoroscopic nephrostography [39]. CEUS nephrostography is particularly suitable in patients with contraindications to iodinated contrast material, or for ill patients as it can be performed at bedside.
Endocavitary CEUS (left) and co-registered grayscale US (right) nephrostography images. UCA can be injected through the drainage catheter to verify the correct placement of the nephrostomy tube. In row (A), the nephrostomy tube is dislodged and there is pooling of microbubble contrast in perinephric spaces (asterisk). CEUS nephrostography following re-insertion of the nephrostomy tube (row B) confirms adequate nephrostomy placement with visualization of microbubble contrast in the renal collecting system (red arrow) and proximal ureter (red arrow head)
CEUS nephrostography. Following a successful nephrostomy insertion, diagnostic evaluation can be obtained with a CEUS nephrostography by introducing microbubbles into the collecting system. A The renal pelvic (asterisk) and the ureter (red arrow heads) can be visualized. B Patent drainage into the bladder can be confirmed by the presence of microbubbles (red block arrow) in the bladder
Biliary intervention
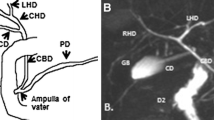
Percutaneous trans-hepatic biliary drainage is commonly performed for the treatment of biliary obstruction. It is critical to determine whether the biliary drainage catheter is positioned adequately to ensure the effectiveness of the drainage. The position of the tip of a biliary drain is often difficult to visualize on conventional US due to bowel gas. A fluoroscopic cholangiography is often required following percutaneous biliary drain insertion or a biliary stent placement. In patients with contraindication to iodinated contrast material or in children in whom ionizing radiation exposure is undesirable, CEUS cholangiography has the potential to offer a practical alternative. CEUS percutaneous trans-hepatic cholangiography was first described in 2009 [40]. Percutaneous access to biliary system can be assisted with CEUS with intravenous administration of UCA to better depict the biliary system, particularly in cases where the biliary system is non-dilated [41]. Endocavitary CEUS with administration of UCA into the biliary system through drainage catheter enables determination of the adequacy of the drainage catheter position [41]. In addition, studies evaluating CEUS cholangiography have shown favorable results in delineating the anatomy of the bile duct tree and confirming bile drainage or the level of impediment to the drainage of bile [21, 42,43,44] (Fig. 10). It has also been reported that CEUS cholangiography can reveal complications associated with the percutaneous trans-hepatic biliary drainage, such as an arterial communication with a percutaneous biliary drainage tube [45], due to its superior temporal and spatial resolution. Furthermore, evaluation of post-surgical complications such as a bile leakage can be performed with CEUS (Fig. 11) but the accuracy for this may be limited on occasion by the presence of bowel gas [41] or pooling of microbubble contrast within a cystic duct remnant. Despite this, CEUS cholangiography following percutaneous drainage could be an advantageous alternative for the assessment of the function of a biliary drain over conventional fluoroscopy in critically ill patients by allowing bedside examination [11].
Endocavitary CEUS cholangiography: Endocavitary CEUS cholangiography with administration of UCA into the biliary system through a drainage catheter demonstrates drainage through the biliary anastomosis into the hepaticojejunostomy (red arrows). Pooling of microbubble contrast (asterisk) is noted near the anastomotic region, raising the suspicion of presence of a small biliary leak. However, caution should be exercised in interpretation of this finding, as pooling of microbubble contrast within a cystic duct remnant may display a similar appearance
Thermal ablation of abdominal tumors
With the advent of thermal ablation technologies, percutaneous ablation has emerged as a viable treatment option as an alternative to surgery in the management of solid abdominal tumors. Frequently utilized ablative mechanisms include radiofrequency ablation (RFA), microwave ablation (MV), cryoablation, particularly for hepatocellular carcinoma [46, 47], hepatic colorectal metastases [48] and renal tumors [49].
Percutaneous ablation of solid abdominal tumors involves the placement of ablation probes into the tumor masses under image guidance. Accurate intra-procedure delineation of tumors and post-ablation evaluation of the ablation zone are fundamental to the effectiveness of treatment. Conventionally, procedural guidance can be achieved with CT or US. US allows multiple planes to perform the procedure in real time. However, even in the most experienced hands, some lesions are poorly visualized on gray-scale US and there can be difficulty in differentiating viable tumors from areas of normal parenchyma or necrosis [50]. CEUS facilitates the treatment as it provides enhancement information that allows lesions inconspicuous on conventional US to be demonstrated during ablation procedures in real time [51]. CEUS also allows improved visualization of residual disease either intra-operatively or immediate post-procedure. With its improved resolution for micro-vascularity, US performed with UCA permits definitive exclusion of any residual vascularity post-ablation [52] (Fig. 12). Abnormal nodular hypervascular region at the peripheral ablation zone on CEUS can be regarded as residual viable tumor [53], and potentially be treated in the same setting [54]. However, in this regard, operators should be mindful of the presence of confounding periablation hyperemia or gas bubbles at the ablation site, which could bring challenges to the interpretation of CEUS appearances immediately post-ablation [55]. Periablation hyperemia often demonstrates a uniform rim of enhancement which, unlike residual tumor, persists throughout the different enhancement phases. Gas bubbles at the ablation site are markedly echogenic on gray-scale US and can be recognized prior to the start of CEUS exam.
A CT (red arrow indicates the ablation zone) and B CEUS images obtained in the immediate post-ablation period following a microwave ablation of a small hepatocellular carcinoma. With its improved temporal and spatial resolution for micro-vascularity, CEUS permits exclusion of any residual vascularity at the ablation zone (asterisk) with confidence
Thermal ablation in liver
Percutaneous thermal ablation therapy is a widely used method for liver tumors, which has been a curative method for small liver cancer treatment [46]. Sonographic guidance is a feasible technique but hepatic tumors can be difficult to be visualized on conventional US, especially after trans-catheter arterial chemo-embolization (TACE). One study [56] reported that tumors could not be visualized on grayscale US in 30% of the patients referred for percutaneous RFA. CEUS has been extensively utilized in thermal ablation procedures for liver tumors that are undetectable via US [57, 58] and evaluation of the ablation zone in the immediately post-procedure [59]. In addition, CEUS using Sonazoid (Daiichi-Sankyo co., Tokyo, Japan) as a UCA in guiding a procedure such as biopsy or RF ablation of liver lesions has been described. Sonazoid microbubbles are taken up by Kupffer cells in the reticuloendothelial system of the liver, with malignant lesions which contain few or no Kupffer cells clearly shown as contrast defects in Kupffer-phase imaging to facilitate intervention [60].
Thermal ablation in kidneys
Recent advances in nephron-sparing procedures have evolved the management of renal tumors. Ablative technologies offer an alternative nephron-sparing treatment to surgery for small renal tumors [61]. Invisibility of renal tumors on conventional US remains the main limiting factor for performing thermal ablation of small renal masses under US guidance [62, 63]. Because of the low resolution of the gray-scale and the low sensitivity for smaller arteries and arterioles, some small isoechoic renal tumors cannot be visualized on conventional US, especially in the deep sections of the renal medulla [64]. The usefulness of CEUS in the assessment of renal anatomy, renal vascularity and focal renal tumors, as well as the assessment of percutaneous ablation therapies has been highlighted in published CEUS guidelines [11, 12]. Due to the excellent imaging of micro-vessels with CEUS, the isoechoic, small renal cell carcinoma and complex residual tumors, even hypo-vascular tumors, can be identified [65]. CEUS was utilized for the guidance percutaneous ablation of renal cell carcinoma in several reports with positive clinical results achieved [66,67,68,69]. Note should be made that CEUS cannot provide information about the excretory function or exclude collecting system injury, as UCA are purely intravascular when administered intravenously.
Post-ablation monitoring
During surveillance following ablation therapy, the ablation scar may be of a similar texture to the surrounding normal tissue on grayscale US. The clarity in assessment of tissue vascularity achieved with CEUS could be used for monitoring effectiveness of ablation therapy and detecting local disease recurrence (Fig. 13). It compares favorably with CT and MRI in follow-up assessment of tumors that were treated with ablation therapy [69,70,71]. CEUS surveillance thus offers an alternative to reduce radiation burden and nephrotoxic contrast medium load for patients in surveillance following tumor ablation therapy.
A Grayscale US and B CEUS images of the right kidney 6 months followup after cryoablation of an upper pole renal cell carcinoma. The cryoablation scar (asterisk) is noted but there are no features suggestive of local recurrence. C Grayscale US and D CEUS images 3 years following cryoablation of the same kidney with recurrence at the ablation site. The recurrence at the ablation scar (t) is of a similar echo reflectivity to the surrounding normal renal parenchyma on grayscale US. The margin (red arrows) of the isoechoic recurrence of renal tumor (t) is better delineated with CEUS due to the excellent ability of CEUS to image the differential vascularity between the tumor and surrounding renal parenchyma
Gastrointestinal application
Endocavitary CEUS offers potential in detecting complications relating to abdominal intervention such as radiologically inserted gastrostomy tubes [72]. UCA can be administered via a gastrostomy tube to confirm correct placement, and exclude presence of a leak (Fig. 14). CEUS could be performed with real-time imaging, and in a variety of clinical settings for instant diagnosis.
Grayscale US and endocavitary CEUS images of a radiologically inserted gastrostomy (RIG) tube. A small hypoechoic collection (asterisk) is present in grayscale US (A). Endocavitary CEUS (B), with administration of microbubble contrast through the gastrostomy tube, showed no accumulation of microbubble contrast in the collection, thus excluding an ongoing leak. Correct placement of the gastrostomy tube is further confirmed with visualization of microbubble contrast on CEUS (D with the corresponding grayscale US (C)) within the gastric cavity (g), which is recognizable due to presence of gastric rugae
Detection of vascular complication following abdominal intervention
Extravasation of intravenous contrast medium is a well-known angiographic and CT finding of active bleeding. Conventional US imaging is able to recognize clots and hematoma but cannot determine whether intra-abdominal bleeding has spontaneously stopped or is still ongoing [73]. Active hemorrhage could be identified on CEUS as extravasation of microbubbles [74] paralleling the known CT and angiographic appearances. CEUS offers additional benefit of the capability to scan the region of interest continuously without radiation burden, allowing exclusion of the theoretical risk of missing a delayed extravasation. In additional to active bleeding, a pseudoaneurysm can develop following an arterial injury which can also be detected with CEUS (Fig. 15). CEUS detection of arterial injuries can also help identify the likely anatomic source of the ongoing bleeding without delay and guide the targeted surgical or angiographic treatment (Fig. 16). CEUS also represents an alternative to contrast-enhanced CT in the followup imaging of arterial injury relating abdominal intervention, arterial complication, without the need for irradiation or administration of iodinated contrast, which may be undesirable for patients with renal insufficiency [75].
Post-renal biopsy pseudoaneurysm. A CEUS demonstrates a pseudoaneurysm (red arrow) within the right kidney. B CEUS performed 1 week following embolization of the pseudoaneurysm demonstrates absence of the pseudoaneurysm and normal perfusion of the surrounding renal parenchyma, confirming the success of the selective embolization procedure
A Grayscale US image following a renal biopsy demonstrated a perinephric hematoma (boundary marked by red arrows), initially thought to be related to bleeding from the kidney. However, the corresponding CEUS (B) clearly demonstrates the pseudoaneurysm (red arrow head) is within the perinephric hematoma rather than within the kidney. c In view of the CEUS appearances, selective angiography of the right intercostal arteries was performed which indeed demonstrated a pseudoaneurysm (red arrow head) arising from a right intercostal artery. No renal arterial injury was demonstrated on angiography. CEUS in this case suggested the possible anatomic site of complicating arterial injury relating to renal biopsy and provided guidance for the subsequent embolization procedure
Pediatric application
US is routinely used as the imaging modality of choice for interventions in the pediatric population because of concern over medical ionizing radiation exposure of children. CEUS is a safe and potentially cost-effective imaging modality for pediatric population [76]. Pediatric CEUS-guided intervention represents a complementary technique for intervention guided by grayscale and color Doppler US. Lumason (Bracco Diagnostics; Monroe Township, NJ) has received regulatory approval for pediatric hepatic use for assessment of pediatric focal liver lesions in the United States [10, 77]. Pediatric CEUS has also been extensively in an “off-label” manner [78] for further indications [79] with promising results, such as in abdominal trauma [75] and in established endocavitary use in voiding urosonography [21]. Furthermore, CEUS-guided pediatric intervention has been reported for chest drainage [80]. The strength of CEUS-guided abdominal intervention over alternative imaging modalities described in current review would also apply to patients in the pediatric population to address specific clinical need, particularly as CEUS negates the need for ionizing radiation or general anesthesia required for alternative therapeutic approach.
Limitations of CEUS
CEUS-guided abdominal intervention shares with conventional US-guided procedures some common causes for potential failure. First, when performing an interventional procedure, the acoustic window often needs to be larger than the window for a diagnostic US exam. Poor acoustic windows, resulting from rib shadows, respiratory movement, limited patient mobility and intervening bowel gas, may all increase procedural difficulty. Second, CEUS and US lack the panoramic properties of CT, and deep positions of abdominal organs or some retroperitoneal areas are not always adequately visualized. Moreover, as a prerequisite, CEUS-guided intervention requires adequate operator experience and expertise in interpreting diagnostic CEUS findings in addition to skills in conventional US-guided intervention. Finally, despite growing experience in the literature on CEUS-guided intervention [33], further comparative clinical trials may be required to fully validate the benefit of CEUS over other imaging modalities for a variety of abdominal interventional procedures.
Conclusion
Contrast-enhanced US, as a natural progression from conventional US, lends itself well to abdominal interventions as it combines traditional advantages of ultrasound guidance with real-time enhancement information without significant side effects. The current state of knowledge suggests CEUS offers a valuable armamentarium for abdominal intervention, not only as a credible alternative to other imaging modalities such as CT, but has the potential to even surpass conventional alternatives and offer solutions for complex logistical or clinical challenges encountered in image-guided abdominal intervention.
References
Schmidbauer J, Remzi M, Memarsadeghi M, et al. (2008) Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 53(5):1003–1011. https://doi.org/10.1016/j.eururo.2007.11.041
Uppot RN, Harisinghani MG, Gervais DA (2010) Imaging-guided percutaneous renal biopsy: rationale and approach. AJR Am J Roentgenol 194(6):1443–1449. https://doi.org/10.2214/AJR.10.4427
Dietrich CF, Lorentzen T, Appelbaum L, et al. (2016) EFSUMB guidelines on interventional ultrasound (INVUS), part III—abdominal treatment procedures (short version). Ultraschall Med 37(1):27–45. https://doi.org/10.1055/s-0035-1553965
Jaffe TA, Nelson RC (2016) Image-guided percutaneous drainage: a review. Abdom Radiol (NY) 41(4):629–636. https://doi.org/10.1007/s00261-016-0649-3
Frederick-Dyer K, Ahmad A, Arora SS, Wile G (2016) Difficult biopsy and drainage: just say yes. Abdom Radiol (NY) 41(4):706–719. https://doi.org/10.1007/s00261-016-0666-2
Sheafor DH, Paulson EK, Simmons CM, DeLong DM, Nelson RC (1998) Abdominal percutaneous interventional procedures: comparison of CT and US guidance. Radiology 207:705–710
Gupta S, Madoff DC (2007) Image-guided percutaneous needle biopsy in cancer diagnosis and staging. Tech Vasc Intervent Radiol 10(2):88–101. https://doi.org/10.1053/j.tvir.2007.09.005
Ma X, Arellano RS, Gervais DA et al (2010) Success of image-guided biopsy for small (≤3 cm) focal liver lesions in cirrhotic and noncirrhotic individuals. J Vasc Interv Radiol JVIR 21(10):1539–1547; quiz 1547. https://doi.org/10.1016/j.jvir.2010.05.025
Beland MD, Sternick LA, Baird GL, et al. (2016) Optimizing modality selection for image-guided procedures: an analysis of the challenges to ultrasound guidance. Abdom Radiol (NY) 41(4):590–599. https://doi.org/10.1007/s00261-016-0637-7
Lumason (2017) Prescribing information. https://doi.org/http://www.braccoimaging.com/us-en/products-and-solutions/contrast-enhanced-ultrasound/lumason/prescribing-information. Accessed 21 Jan 2017.
Piscaglia F, Nolsoe C, Dietrich CF, et al. (2012) The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 33(1):33–59. https://doi.org/10.1055/s-0031-1281676
Sidhu PS, Brabrand K, Cantisani V, et al. (2015) EFSUMB guidelines on interventional ultrasound (INVUS), part II. Ultraschall Med 36(6):E15–E35. https://doi.org/10.1055/s-0035-1554036
Duncan JRTD (2015) Improving performance during image-guided procedures. J Patient Saf 11(4):230–236. https://doi.org/10.1097/PTS.0000000000000115
Quaia E (2007) Microbubble ultrasound contrast agents: an update. Eur Radiol 17(8):1995–2008. https://doi.org/10.1007/s00330-007-0623-0
Harvey CJ, Blomley MJK, Eckersley RJ, Cosgrove DO (2001) Developments in ultrasound contrast media. Eur Radiol 11:675–689
Piscaglia F, Bolondi L, Italian Society for Ultrasound in M, Biology Study Group on Ultrasound Contrast A (2006) The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 32(9):1369–1375. https://doi.org/10.1016/j.ultrasmedbio.2006.05.031
Heinzmann A, Muller T, Leitlein J, et al. (2012) Endocavitary contrast enhanced ultrasound (CEUS)–work in progress. Ultraschall Med 33(1):76–84. https://doi.org/10.1055/s-0031-1299056
Muller T, Leitlein J, Kubicka S, Heinzmann A (2015) Endocavitary contrast-enhanced ultrasound: a technique whose time has come? J Clin Ultrasound JCU 43(2):71–80. https://doi.org/10.1002/jcu.22250
Chenia F, Hofmeyr GJ, Moolla S, Oratis P (1997) Sonographic hydrotubation using agitated saline: a new technique for improving fallopian tube visualization. Br J Radiol 70(836):833–836. https://doi.org/10.1259/bjr.70.836.9486049
Hanbury DC, Coulden RA, Farman P, Sherwood T (1990) Ultrasound cystography in the diagnosis of vesicoureteric reflux. Br J Urol 65(3):250–253
Luyao Z, Xiaoyan X, Huixiong X, et al. (2012) Percutaneous ultrasound-guided cholangiography using microbubbles to evaluate the dilated biliary tract: initial experience. Eur Radiol 22(2):371–378. https://doi.org/10.1007/s00330-011-2265-5
Darge K (2008) Voiding urosonography with ultrasound contrast agents for the diagnosis of vesicoureteric reflux in children. I. Procedure. Pediatric Radiol 38(1):40–53. https://doi.org/10.1007/s00247-007-0529-7
Zhou L, Zhang X, Chen X, et al. (2012) Value of three-dimensional hysterosalpingo-contrast sonography with SonoVue in the assessment of tubal patency. Ultrasound Obstet Gynecol 40(1):93–98. https://doi.org/10.1002/uog.11085
Goddi A, Novario R, Tanzi F, Di Liberto R, Nucci P (2004) In vitro analysis of ultrasound second generation contrast agent diluted in saline solution. Radiol Med 107(5–6):569–579
Bartolotta TV, Taibbi A, Midiri M, Lagalla R (2009) Focal liver lesions: contrast-enhanced ultrasound. Abdom Imaging 34(2):193–209. https://doi.org/10.1007/s00261-008-9378-6
Meloni MF, Andreano A, Laeseke PF, et al. (2008) Contrast-enhanced ultrasonographic findings in a brucellar hepatic abscess. J Ultrasound Med 27(10):1511–1515
Kishina M, Koda M, Tokunaga S, et al. (2015) Usefulness of contrast-enhanced ultrasound with Sonazoid for evaluating liver abscess in comparison with conventional B-mode ultrasound. Hepatol Res 45(3):337–342. https://doi.org/10.1111/hepr.12347
Sainani NI, Arellano RS, Shyn PB, et al. (2013) The challenging image-guided abdominal mass biopsy: established and emerging techniques ‘if you can see it, you can biopsy it’. Abdom Imaging 38(4):672–696. https://doi.org/10.1007/s00261-013-9980-0
Sparchez Z, Radu P, Zaharia T, et al. (2010) Contrast enhanced ultrasound guidance: a new tool to improve accuracy in percutaneous biopsies. Med Ultrasonogr 12(2):133–138
Yoon SH, Lee KH, Kim SY, et al. (2010) Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol 20:2047–2056. https://doi.org/10.1007/s00330-010-1757-z
Mao F, Dong Y, Ji Z, Cao J, Wang WP (2017) Contrast-enhanced ultrasound guided biopsy of undetermined abdominal lesions: a multidisciplinary decision-making approach. Biomed Res Int 2017:8791259. https://doi.org/10.1155/2017/8791259
Spârchez Z, Radu P, Kacsó G, et al. (2012) Contrast-enhanced ultrasound guided biopsy of super cial toraco-abdominal and neck lesions. Initial experience in 20 patients. Med Ultrasonogr 14(4):288–293
Huang DY, Yusuf GT, Daneshi M, et al. (2017) Contrast-enhanced US-guided interventions: improving success rate and avoiding complications using US contrast agents. RadioGraphics 37(2):652–664. https://doi.org/10.1148/rg.2017160123
Wang J, Zhou D, Xie X, Shen P, Zeng Y (2015) Utility of contrast-enhanced ultrasound with SonoVue in biopsy of small subpleural nodules. Int J Clin Exp Med 8(9):15991–15998
Ori Y, Neuman H, Chagnac A, et al. (2002) Using the automated biopsy gun with real-time ultrasound for native renal biopsy. Isr Med Assoc J 4(9):698–701
Montvilas P, Solvig J, Johansen TE (2011) Single-centre review of radiologically guided percutaneous nephrostomy using “mixed” technique: success and complication rates. Eur J Radiol 80(2):553–558. https://doi.org/10.1016/j.ejrad.2011.01.109
Liu BX, Huang GL, Xie XH, et al. (2017) Contrast-enhanced US-assisted percutaneous nephrostomy: a technique to increase success rate for patients with nondilated renal collecting system. Radiology 285(1):293–301
Chi T, Usawachintachit M, Weinstein S, et al. (2017) Contrast enhanced ultrasound as a radiation-free alternative to fluoroscopic nephrostogram for evaluating ureteral patency. J Urol . https://doi.org/10.1016/j.juro.2017.07.074
Cui XW, Ignee A, Maros T, et al. (2016) Feasibility and usefulness of intra-cavitary contrast-enhanced ultrasound in percutaneous nephrostomy. Ultrasound Med Biol 42(9):2180–2188. https://doi.org/10.1016/j.ultrasmedbio.2016.04.015
Ignee A, Baum U, Schuessler G, Dietrich CF (2009) Contrast-enhanced ultrasound-guided percutaneous cholangiography and cholangiodrainage (CEUS-PTCD). Endoscopy 41(8):725–726. https://doi.org/10.1055/s-0029-1214956
Mao R, Xu EJ, Li K, Zheng RQ (2010) Usefulness of contrast-enhanced ultrasound in the diagnosis of biliary leakage following T-tube removal. J Clin Ultrasound JCU 38(1):38–40. https://doi.org/10.1002/jcu.20622
Xu EJ, Zheng RQ, Su ZZ, et al. (2012) Intra-biliary contrast-enhanced ultrasound for evaluating biliary obstruction during percutaneous transhepatic biliary drainage: a preliminary study. Eur J Radiol 81(12):3846–3850. https://doi.org/10.1016/j.ejrad.2012.06.025
Meacock LM, Sellars ME, Sidhu PS (2010) Evaluation of gallbladder and biliary duct disease using microbubble contrast-enhanced ultrasound. Br J Radiol 83(991):615–627. https://doi.org/10.1259/bjr/60619911
Chen LD, Xu HX, Xie XY, et al. (2010) Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol 20(3):743–753. https://doi.org/10.1007/s00330-009-1599-8
Daneshi M, Rajayogeswaran B, Peddu P, Sidhu PS (2014) Demonstration of an occult biliary-arterial fistula using percutaneous contrast-enhanced ultrasound cholangiography in a transplanted liver. J Clin Ultrasound 42(2):108–111. https://doi.org/10.1002/jcu.22048
Bruix J, Hessheimer AJ, Forner A, et al. (2006) New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene 25(27):3848–3856. https://doi.org/10.1038/sj.onc.1209548
Livraghi T, Goldberg SN, Lazzaroni S, et al. (2000) Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 214(3):761–768
Gillams AR, Lees WR (2009) Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 19(5):1206–1213. https://doi.org/10.1007/s00330-008-1258-5
Dominguez-Escrig JL, Sahadevan K, Johnson P (2008) Cryoablation for small renal masses. Adv Urol, Article ID 479495. https://doi.org/10.1155/2008/479495
Claudon M, Dietrich CF, Choi BI, et al. (2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 39(2):187–210. https://doi.org/10.1016/j.ultrasmedbio.2012.09.002
Chen C, Liang P, Yu J, et al. (2016) Contrast-enhanced ultrasound-guided percutaneous microwave ablation of renal cell carcinoma that is inconspicuous on conventional ultrasound. Int J Hyperth 32(6):607–613. https://doi.org/10.3109/02656736.2016.1172118
Nishigaki Y, Hayashi H, Tomita E, et al. (2015) Usefulness of contrast-enhanced ultrasonography using Sonazoid for the assessment of therapeutic response to percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res 45(4):432–440. https://doi.org/10.1111/hepr.12370
Minami Y, Kudo M, Kawasaki T, et al. (2004) Treatment of hepatocellular carcinoma with percutaneous radiofrequency ablation: usefulness of contrast harmonic sonography for lesions poorly defined with B-mode sonography. AJR Am J Roentgenol 183(1):153–156
Dohmen T, Kataoka E, Yamada I, et al. (2012) Efficacy of contrast-enhanced ultrasonography in radiofrequency ablation for hepatocellular carcinoma. Intern Med 51(1):1–7. https://doi.org/10.2169/internalmedicine.51.6042
Lekht I, Gulati M, Nayyar M, et al. (2016) Role of contrast-enhanced ultrasound (CEUS) in evaluation of thermal ablation zone. Abdom Radiol (NY) . https://doi.org/10.1007/s00261-016-0700-4
Rhim H, Lee MH, Kim Y, et al. (2008) Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR Am J Roentgenol 190(5):1324–1330. https://doi.org/10.2214/AJR.07.2970
Liu F, Yu X, Liang P, et al. (2011) Contrast-enhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperth 27(6):555–562. https://doi.org/10.3109/02656736.2011.564262
Minami Y, Kudo M, Chung H, et al. (2007) Contrast harmonic sonography-guided radiofrequency ablation therapy versus B-mode sonography in hepatocellular carcinoma: prospective randomized controlled trial. Am J Roentgenol 188(2):489–494. https://doi.org/10.2214/ajr.05.1286
Numata K, Morimoto M, Ogura T, et al. (2008) Ablation therapy guided by contrast-enhanced sonography with Sonazoid for hepatocellular carcinoma lesions not detected by conventional sonography. J Ultrasound Med 27(3):395–406
Kim TK, Khalili K, Jang HJ (2015) Local ablation therapy with contrast-enhanced ultrasonography for hepatocellular carcinoma: a practical review. Ultrasonography 34(4):235–245. https://doi.org/10.14366/usg.15018
Wehle MJ, Thiel DD, Petrou SP, et al. (2004) Conservative management of incidental contrast-enhancing renal masses as safe alternative to invasive therapy. Urology 64(1):49–52. https://doi.org/10.1016/j.urology.2004.02.026
Park BK, Kim CK, Choi HY, et al. (2010) Limitation for performing ultrasound-guided radiofrequency ablation of small renal masses. Eur J Radiol 75(2):248–252. https://doi.org/10.1016/j.ejrad.2009.03.050
Veltri A, Garetto I, Pagano E, et al. (2009) Percutaneous RF thermal ablation of renal tumors: is US guidance really less favorable than other imaging guidance techniques? Cardiovasc Intervent Radiol 32(1):76–85. https://doi.org/10.1007/s00270-008-9414-5
Forman HP, Middleton WD, Melson GL, McClennan BL (1993) Hyperechoic renal cell carcinomas: increase in detection at US. Radiology 188(2):431–434
Tamai H, Takiguchi Y, Oka M, et al. (2005) Contrast-enhanced ultrasonography in the diagnosis of solid renal tumors. J Ultrasound Med 24:1635–1640
Carrafiello G, Mangini M, Fontana F, et al. (2010) Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Intervent Radiol 33(2):367–374. https://doi.org/10.1007/s00270-009-9745-x
Zhao X, Wang W, Zhang S, et al. (2012) Improved outcome of percutaneous radiofrequency ablation in renal cell carcinoma: a retrospective study of intraoperative contrast-enhanced ultrasonography in 73 patients. Abdom Imaging 37(5):885–891
Lackey L, Peterson C, Barr RG (2012) Contrast-enhanced ultrasound-guided radiofrequency ablation of renal tumors. Ultrasound Q 28(4):269–274. https://doi.org/10.1097/RUQ.0b013e318274de66
Meloni MF, Andreano A, Franza E, Passamonti M, Lazzaroni S (2012) Contrast enhanced ultrasound: should it play a role in immediate evaluation of liver tumors following thermal ablation? Eur J Radiol 81(8):e897–902. https://doi.org/10.1016/j.ejrad.2012.05.002
Lu MD, Yu XL, Li AH, et al. (2007) Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 33(11):1736–1749. https://doi.org/10.1016/j.ultrasmedbio.2007.05.004
Morimoto M, Nozawa A, Numata K, et al. (2005) Evaluation using contrast-enhanced harmonic gray scale sonography after radio frequency ablation of small hepatocellular carcinoma: sonographic-histopathologic correlation. J Ultrasound Med 24(3):273–283
Lewis D, Ampong MA, Rio A, et al. (2009) Mushroom-cage gastrostomy tube placement in patients with amyotrophic lateral sclerosis: a 5-year experience in 104 patients in a single institution. Eur Radiol 19(7):1763–1771. https://doi.org/10.1007/s00330-009-1307-8
Liu JB, Merton DA, Goldberg BB, et al. (2002) Contrast-enhanced two- and three-dimensional sonography for evaluation of intra-abdominal hemorrhage. J Ultrasound Med 21(2):161–169
Catalano O, Lobianco R, Cusati B, Siani A (2005) Contrast-enhanced sonography for diagnosis of ruptured abdominal aortic aneurysm. AJR Am J Roentgenol 184(2):423–427
Durkin N, Deganello A, Sellars ME, et al. (2016) Post-traumatic liver and splenic pseudoaneurysms in children: diagnosis, management, and follow-up screening using contrast enhanced ultrasound (CEUS). J Pediatr Surg 51(2):289–292. https://doi.org/10.1016/j.jpedsurg.2015.10.074
Yusuf GT, Sellars ME, Deganello A, Cosgrove DO, Sidhu PS (2017) Retrospective analysis of the safety and cost implications of pediatric contrast-enhanced ultrasound at a single center. AJR Am J Roentgenol 208(2):446–452. https://doi.org/10.2214/AJR.16.16700
Anupindi SA, Biko DM, Ntoulia A, et al. (2017) Contrast-enhanced US assessment of focal liver lesions in children. Radiographics 37(6):1632–1647. https://doi.org/10.1148/rg.2017170073
Sidhu PS, Cantisani V, Deganello A, et al. (2017) Role of contrast-enhanced ultrasound (CEUS) in paediatric practice: an EFSUMB position statement. Ultraschall Med 38(1):33–43. https://doi.org/10.1055/s-0042-110394
Rafailidis V, Deganello A, Watson T, Sidhu PS, Sellars ME (2017) Enhancing the role of paediatric ultrasound with microbubbles: a review of intravenous applications. Br J Radiol 90(1069):20160556. https://doi.org/10.1259/bjr.20160556
Deganello A, Rafailidis V, Sellars ME, et al. (2017) Intravenous and intracavitary use of contrast-enhanced ultrasound in the evaluation and management of complicated pediatric pneumonia. J Ultrasound Med . https://doi.org/10.1002/jum.14269
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflict of interest
MD and RR declare that they have no potential conflict of interest. GY has received lecture fees from Bracco. DH, AD and MS has received fees from Bracco for providing a training workshop on CEUS. PS has received lecture fees from Bracco, Siemens, Samsung, Philips, and Hitachi.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Huang, D.Y., Yusuf, G.T., Daneshi, M. et al. Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom Radiol 43, 960–976 (2018). https://doi.org/10.1007/s00261-018-1473-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1473-8