Abstract
Purpose
FDG-positive neuroendocrine tumors (NETs) have a poorer prognosis and exhibit shorter response duration to peptide receptor radionuclide therapy (PRRT). The aim of this prospective phase II study was to evaluate the efficacy and toxicity of PRRT with 177Lu-DOTATATE associated with metronomic capecitabine as a radiosensitizer agent in patients with advanced progressive FDG-positive gastro-entero-pancreatic (GEP) NETs.
Patients and methods
Patients with advanced somatostatin receptor- and FDG-positive G1-G3 GEP-NETs (Ki67 < 55%) were treated with a cumulative activity of 27.5 GBq of 177Lu-DOTATATE divided in five cycles of 5.5 GBq each every 8 weeks. Capecitabine (1000–1500 mg daily) was administered orally in the inter-cycle period between 177Lu-DOTATATE treatments. Prior to commencing capecitabine, all patients were triaged with the dihydropyrimidine dehydrogenase (DPD) test. Only DPD-proficient individuals were enrolled. The primary objectives were disease control rate (DCR) and safety. Secondary aims included progression-free (PFS) and overall survival (OS). Treatment response was assessed per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Toxicity was assessed by Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Results
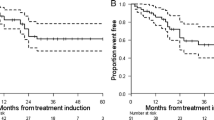
From August 2015 to December 2016, 37 subjects were consecutively enrolled. A total of 25 (68%) were affected by pancreatic neuroendocrine tumors (P-NETs), and 12 (32%) had gastrointestinal neuroendocrine tumors (GI-NETs). By grading (WHO 2010 classification), 12 patients (32%) had G1 (Ki67 ≤ 2%), 22 (59%) had G2 (3% < Ki67 ≤ 20%), and 3 patients (9%) had G3 (Ki67 > 20%) NETs. Grade 3 (G3) or 4 (G4) hematological toxicity occurred in 16.2% of patients. Other G3-G4 adverse events were diarrhea in 5.4% of cases and asthenia in 5.4%. No renal toxicity was observed for the duration of follow-up. In 37 patients, 33 were evaluable for response. Objective responses included partial response (PR) in 10 patients (30%) and stable disease (SD) in 18 patients (55%), with a DCR of 85%. The median follow-up was 38 months (range 4.6–51.1 months). The median PFS was 31.4 months (17.6–45.4), and mOS was not reached.
Conclusions
This study demonstrated that the combination of PRRT with 177Lu-DOTATATE and metronomic capecitabine is active and well tolerated in patients with aggressive FDG-positive G1-G3 GEP-NETs. These data constitute the basis for a randomized study of PPRT alone vs. PRRT plus metronomic capecitabine.



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259–77.
Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA ,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30.
Paganelli G, Sansovini M, Ambrosetti A, et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging. 41:1845–51.
Sansovini M, Severi S, Ianniello A, et al. Long-term follow-up and role of FDG-PET in advanced pancreatic neuroendocrine patients treated with 177Lu-DOTATATE. Eur J Nucl Med Mol Imaging. 2017;44:490–9.
Strosberg J, El-Haddad G, Wolin E, et al. NETTER-1 trial investigators. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35.
Hofman MS, Hicks RJ. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med. 2012;14:71–81.
Panagiotidis E, Alshammari A, Michopoulou S, et al. Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. J Nucl Med. 2017;58:91–6.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Bahri H, Laurence L, Edeline J, et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med. 2014;55:1786–90.
Binderup T, Knigge U, Loft A, et al. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–85.
Severi S, Nanni O, Bodei L, et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:881–8.
Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177luoctreotate in combination with capecitabine and temozolomide in advanced lowgrade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27:561–9.
Claringbold PG, Turner JH. Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-octreotate-capecitabine-temozolomide radiopeptide chemotherapy. Neuroendocrinology. 2016;103:432–9.
Ballal S, Yadav MP, Damle NA, et al. Concomitant 177Lu-DOTATATE and capecitabine therapy in patients with advanced neuroendocrine Tumors: a long-term-outcome, toxicity, survival, and quality-of-life study. Clin Nucl Med. 2017;42:e457–66.
Kesavan M, Claringbold PG, Turner JH. Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology. 2014;99:108–17.
Kashyap R, Hofman MS, Michael M, et al. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2015;42:176–85.
Claringbold PG, Brayshaw PA, Price RA, et al. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:302–11.
Krenning EP, Bakker WH, Breeman WA, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1:242–4.
Bosman F, Carneiro F, Hruban R, et al. World Health Organization Classification of Tumours. Pathology and Genetics of tumors of the digestive system. Lyon: IARC Press; 2010.
Breeman WA, de Blois E, Bakker WH. Radiolabeling DOTA peptides with 90Yand 177Lu to a high specific activity. In: Chinol M, Paganelli G, editors. Radionuclide peptide cancer therapy. New York: Taylor & Francis; 2006. p. 119–26.
Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35.
Chan DL, Pavlakis N, Schembri GP, et al. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics. 2017;7:1149–58.
WHO Classification of Tumours Editorial Board. Digestive System Tumours. 5. Lyon: International Agency for Research on Cancer; 2019. p. 16–8.
Lloyd RV, Osamura RY, Kloppel G. WHO classification of tumours of endocrine organs, chapter 6. 4th ed. Lyon: International Agency for Research on Cancer; 2017. p. 210–39.
Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72.
Pencharz D, Gnanasegaran G, Navalkissoor S. Theranostics in neuroendocrine tumours: somatostatin receptor imaging and therapy. Br J Radiol. 2018;91:20180108.
Zerizer I, Al-Nahhas A, Towey D, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging. 2012;39:1391–9.
Oh S, Prasad V, Lee DS, et al. Effect of peptide receptor radionuclide therapy on somatostatin receptor status and glucose metabolism in neuroendocrine tumors: intraindividual comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging Int J Mol Imaging. 2011;2011:524130.
Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–73.
Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19.
Meulendijks D, Cats A, Beijnen JH, et al. Improving safety of fluoropyrimidine chemotherapy by individualizing treatment based on dihydropyrimidine dehydrogenase activity - ready for clinical practice? Cancer Treat Rev. 2016;50:23–34.
Collovà E, Sebastiani F, De Matteis E, et al. Use of metronomic chemotherapy in oncology: results from a national Italian survey. Tumori. 2011;97:454–8.
Laquente B, Vinals F, Germa JR. Metronomic chemotherapy: an antiangiogenic scheduling. Clin Transl Oncol. 2007;9:93–8.
Bongiovanni A, Riva N, Calpona S, et al. Metronomic capecitabine in gastroenteropancreatic neuroendrocrine tumors: a suitable regimen and review of the literature. Onco Targets Ther. 2014;7:1919–26.
Zhang J, Liu Q, Singh A, et al. Prognostic value of 18F-FDG PET/CT in a large cohort of 495 patients with advanced metastatic neuroendocrine neoplasms (NEN) treated with peptide receptor radionuclide therapy (PRRT). J Nucl Med. 2020;119:241414.
Zemczak A, Kołodziej M, Gut P, et al. Effect of peptide receptor radionuclide therapy (PRRT) with tandem isotopes - [90Y]Y/[177Lu]Lu-DOTATATE in patients with disseminated neuroendocrine tumours depending on [18F]FDG PET/CT qualification in polish multicentre experience - do we need [18F]FDG PET/CT for qualification to PRRT? Endokrynol Pol. 2020;71:240–8.
Acknowledgments
The authors thank Gráinne Tierney for editorial assistance.
Funding
This work is partially supported by Fondazione AIRC per la Ricerca sul Cancro and Italian Ministry of Health.
Author information
Authors and Affiliations
Contributions
Study concept and design, Giovanni Paganelli and Lisa Bodei; provision of study materials or patients, Silvia Nicolini, Alberto Bongiovanni, Maddalena Sansovini, Stefano Severi, Ilaria Grassi, Toni Ibrahim, Chiara Maria Grana, Valentina Di Iorio, and Corrado Cittanti; diagnostic and therapeutic imaging, Paola Caroli and Danila Diano; pathology review, Federica Pieri; quality control and gamma camera calibration, Anna Sarnelli; data management, Manuela Monti; analysis and interpretation of data, Giovanni Paganelli and Silvia Nicolini; drafting of the manuscript, Silvia Nicolini, Lisa Bodei, and Giovanni Paganelli; critical revision of the manuscript for important intellectual content, Giovanni Paganelli and Lisa Bodei. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the Ethics Committee of the Area Vasta Romagna-IRST (Approval No. 6929/2014 of December 17, 2014) and by the competent authorities and was conducted in full conformity with the current revision of the Declaration of Helsinki, the current ICH Guidelines for Good Clinical Practice, the Directive 2001/20/EEC, and other relevant current local legislation.
Statement of informed consent
All patients gave written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – General
Rights and permissions
About this article
Cite this article
Nicolini, S., Bodei, L., Bongiovanni, A. et al. Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging 48, 3260–3267 (2021). https://doi.org/10.1007/s00259-021-05236-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05236-z