Abstract
Purpose
[18F]FDG-PET and [11C]PIB-PET are validated as neurodegeneration and amyloid biomarkers of Alzheimer’s disease (AD). We used a PET staging system based on the 2018 NIA-AA research framework to compare the proportion of amyloid positivity (A+) and hypometabolism ((N)+) in cases of mild probable AD, amnestic mild cognitive impairment (aMCI), and healthy controls, incorporating an additional classification of abnormal [18F]FDG-PET patterns and investigating the co-occurrence of such with A+, exploring [18F]FDG-PET to generate hypotheses in cases presenting with clinical-biomarker “mismatches.”
Methods
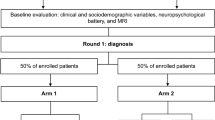
Elderly individuals (N = 108) clinically classified as controls (N = 27), aMCI (N = 43) or mild probable AD (N = 38) were included. Authors assessed their A(N) profiles and classified [18F]FDG-PET neurodegenerative patterns as typical or non-typical of AD, performing re-assessments of images whenever clinical classification was in disagreement with the PET staging (clinical-biomarker “mismatches”). We also investigated associations between “mismatches” and sociodemographic and educational characteristics.
Results
AD presented with higher rates of A+ and (N)+. There was also a higher proportion of A+ and (N)+ individuals in the aMCI group in comparison to controls, however without statistical significance regarding the A staging. There was a significant association between amyloid positivity and AD (N)+ hypometabolic patterns typical of AD. Non-AD (N)+ hypometabolism was seen in all A− (N)+ cases in the mild probable AD and control groups and [18F]FDG-PET patterns classified such individuals as “SNAP” and one as probable frontotemporal lobar degeneration. All A− (N)− cases in the probable AD group had less than 4 years of formal education and lower socioeconomic status (SES).
Conclusion
The PET-based staging system unveiled significant A(N) differences between AD and the other groups, whereas aMCI and controls had different (N) staging, explaining the cognitive impairment in aMCI. [18F]FDG-PET could be used beyond simple (N) staging, since it provided alternative hypotheses to cases with clinical-biomarker “mismatches.” An AD hypometabolic pattern correlated with amyloid positivity. Low education and SES were related to dementia in the absence of biomarker changes.


Similar content being viewed by others
Change history
26 June 2020
In the last paragraph of the subsession “Recruitment of the study population and clinical Evaluation” (Material and methods session).
References
Jack C, Bennett D, Blennow K, Carrillo M, Dunn B, Haeberlein S, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Jack CR Jr, Wiste HJ, Knopman DS, Vemuri P, Mielke MM, Weigand SD, Senjem ML, Gunter JL, Lowe V, Gregg BE, Pankratz VS, Petersen RC. Rates of β-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82(18):1605–12.
Jack C, Bennett D, Blennow K, Carrillo M, Feldman H, Frisoni G, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47.
Pike, Savage, Villemagne, Ng, Moss, Maruff, et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–44.
Mormino E, Betensky R, Hedden T, Schultz A, Amariglio R, Rentz D, et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71:1379–85.
Hedden T, Oh H, Younger A, Patel T. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–8.
Knopman D, Haeberlein S, Carrillo M, Hendrix J, Kerchner G, Margolin R, et al. The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: perspectives from the research roundtable. Alzheimer’s Dement. 2018;14:563–75.
Klunk W, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt D, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19.
Camus, Payoux, Barré, Desgranges, Voisin, Tauber, et al. Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur J Nucl Med Mol Imaging. 2012;39:621–31.
Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, et al. Simplified quantification of Pittsburgh compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–72.
Clark C, Pontecorvo M, Beach T, Bedell B, Coleman R, Doraiswamy P, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78.
La Joie R, Ayakta N, Seeley WW, Borys E, Boxer AL, DeCarli C, Doré V, Grinberg LT, Huang E, Hwang JH, Ikonomovic MD, Jack C Jr, Jagust WJ, Jin LW, Klunk WE, Kofler J, Lesman-Segev OH, Lockhart SN, Lowe VJ, Masters CL, Mathis CA, McLean CL, Miller BL, Mungas D, O'Neil JP, Olichney JM, Parisi JE, Petersen RC, Rosen HJ, Rowe CC, Spina S, Vemuri P, Villemagne VL, Murray ME, Rabinovici GD. Multisite study of the relationships between antemortem [11C]PIB-PET Centiloid values and postmortem measures of Alzheimer's disease neuropathology. Alzheimers Dement. 2019;15:205–216.
Villemagne V. Selective Tau Imaging:Der Stand der Dinge. J Nucl Med. 2017;59:175–6.
Burdette M, Borght V, Tran DD, Kuhl DE. Alzheimer disease: improved visual interpretation of PET images by using three-dimensional stereotaxic surface projections. Radiology. 1996;198:837–43.
Minoshima S, Giordani B, Berent S, Frey K, Foster N, Kuhl D. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94.
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol I. 2003;30:1104–13.
Cerami C, Rosa P, Magnani G, Santangelo R, Marcone A, Cappa S, et al. Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. Neuroimage Clin. 2015;7:187–94.
Toussaint P-J, Perlbarg V, Bellec P, Desarnaud S, Lacomblez L, Doyon J, et al. Resting state FDG-PET functional connectivity as an early biomarker of Alzheimer’s disease using conjoint univariate and independent component analyses. Neuroimage. 2012;63:936–46.
Silverman D, Small G, Chang C, Lu C, de Aburto M, Chen W, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–7.
Perani D, Cerami C, Caminiti S, Santangelo R, Coppi E, Ferrari L, et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging. 2016;43:499–508.
Petrie E, Cross D, Galasko D, Schellenberg G, Raskind M, Peskind E, et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–7.
Ossenkoppele R, Prins N, Pijnenburg Y, Lemstra A, van der Flier W, Adriaanse S, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9:414–21.
McKhann G, Knopman D, Chertkow H, Hyman B, Jack C, Kawas C, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Del-Ben C, Vilela J, Crippa J, Hallak J, Labate C, Zuardi A. Confiabilidade da “Entrevista Clínica Estruturada para o DSM-IV - Versão Clínica” traduzida para o português. Rev Bras Psiquiatr. 2001;23:156–9.
Blessed G, Tomlinson B, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;144:797–811.
Folstein M, Foltstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Petersen RC. Mild cognitive impairment. N Engl J Med. 2011;364:2234.
Albert M, DeKosky S, Dickson D, Dubois B, Feldman H, Fox N, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
ABA. Critério de classificação sócio-econômica. 2007. http://www.aba.com.br/wp-content/uploads/content/7727632a373615b34f2a5726fcc5c9e2.pdf - content in portuguese). Assessed 7 Mar 2018.
Faria DP, Duran FL, Squarzoni P, Coutinho AM, Garcez AT, Santos PP, Brucki SM, de Oliveira MO, Trés ES, Forlenza OV, Nitrini R, Buchpiguel CA, Busatto Filho G. Topography of 11C-Pittsburgh compound B uptake in Alzheimer's disease: a voxel-based investigation of cortical and white matter regions. Braz J Psychiatry. 2019;41:101-111.
Group J-A, Yamane T, Ishii K, Sakata M, Ikari Y, Nishio T, et al. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of 11C-PiB PET amyloid images of the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:850–7.
Leuzy A, Chiotis K, Hasselbalch S, Rinne J, Mendonça A, Otto M, et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain. 2016;139:2540–53.
Klunk WE, Koeppe R, Price J, Benzinger T, Devous M, Jagust W, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1–15.e4.
Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Gunter JL, Senjem ML, Jones DT, Kantarci K, Machulda MM, Mielke MM, Roberts RO, Vemuri P, Reyes DA, Petersen RC. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205-21.
Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–33.
Jack CR Jr, Therneau TM, Weigand SD, Wiste HJ, Knopman DS, Vemuri P. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging–Alzheimer’s Association Research Framework. JAMA Neurol. 2019. https://doi.org/10.1001/jamaneurol.2019.1971.
Mosconi L, Tsui W, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49:390–8.
Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2008;39:619–33.
Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz H-G, et al. SPM-based count normalization provides excellent discrimination of mild Alzheimer’s disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009;44:43–50.
Dukart J, Mueller K, Horstmann A, Vogt B, Frisch S, Barthel H, et al. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. Neuroimage. 2010;49:1490–5.
Küntzelmann A, Guenther T, Haberkorn U, Essig M, Giesel F, Henze R, et al. Impaired cerebral glucose metabolism in prodromal Alzheimer’s disease differs by regional intensity normalization. Neurosci Lett. 2013;534:12–7.
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–48.
Teune L, Bartels A, de Jong B, Willemsen A, Eshuis S, de Vries J, et al. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord. wiley. 2010;25:2395–404.
Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13.
Dyck C. Anti-amyloid-β monoclonal antibodies for Alzheimer’s disease: pitfalls and promise. Biol Psychiatry. 2018;83:311–9.
Rabinovici G, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, et al. Association of Amyloid Positron Emission Tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321:1286–94.
Wang L, Benzinger T, Su Y, Christensen J, Friedrichsen K, Aldea P, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between β-amyloid and tauopathy. Jama Neurol. 2016;73:1070.
McRae-McKee K, Udeh-Momoh CT, Price G, Bajaj S, de Jager CA, et al. Perspective: clinical relevance of the dichotomous classification of Alzheimer’s disease biomarkers: should there be a “gray zone”? Alzheimers Dement. 2019;15:1348–56.
Ismail R, Parbo P, Hansen KV, Schaldemose JL, Dalby RB, Tietze A, et al. Abnormal amyloid load in mild cognitive impairment: the effect of reducing the PiB-PET threshold. J Neuroimaging. 2019;00:1–7.
Silverman D. Brain 18F-FDG PET in the diagnosis of neurodegenerative dementias: comparison with perfusion SPECT and with clinical evaluations lacking nuclear imaging. J Nucl Med. 2004;45:594–607.
Landau S, Harvey D, Madison C, Koeppe R, Reiman E, Foster N, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18.
Coutinho A, Porto F, Duran F, Prando S, Ono C, Feitosa E, et al. Brain metabolism and cerebrospinal fluid biomarkers profile of non-amnestic mild cognitive impairment in comparison to amnestic mild cognitive impairment and normal older subjects. Alzheimers Res Ther. 2015;7:1–10.
Murray M, Senjem M, Petersen R, Hollman J, Preboske G, Weigand S, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–85.
Reed B, Eberling J, Mungas D, Weiner M, Kramer J, Jagust W. Effects of white matter lesions and lacunes on cortical function. Arch Neurol. 2004;61:1545–50.
Jack C, Lowe V, Senjem M, Weigand S, Kemp B, Shiung M, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80.
Jicha GA, Nelson PT. Hippocampal sclerosis, argyrophilic grain disease, and primary age-related tauopathy. Continuum (Minneap Minn). 2019;25:208–33.
Nelson PT, Dickson DW, Trojanowski JQ, Jack CR Jr, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142:1503–27.
Rusmaully J, Dugravot A, Moatti JP, Marmot MG, Elbaz A, Kivimaki M, et al. Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: a cohort study. PLoS Med. 2017;14:e1002334.
Sattler C, Toro P, Schönknecht P, Schröder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012;196:90–952011.
Schwartz CE, Zhang J, Stucky BD, Michael W, Rapkin BD. Is the link between socioeconomic status and resilience mediated by reserve-building activities: mediation analysis of web-based cross-sectional data from chronic medical illness patient panels. BMJ Open. 2019;9:e025602.
Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12.
Tucker AM, Stern Y. Cognitive reserve in aging. Curr Alzheimer Res. 2011;8:354–60.
Garibotto, Borroni, Kalbe, Herholz, Salmon. Education and occupation as proxies for reserve in aMCI converters and AD FDG-PET evidence. Neurology. 2008;71:1342–9.
Ewers M, Insel P, Stern Y, Weiner M. (ADNI) F. Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology. 2013;80:1194–201.
Hoenig MC, Bischof GN, Onur OA, Kukolja J, Jessen F, Fliessbach K, et al. Level of education mitigates the impact of tau pathology on neuronal function. Eur J Nucl Med Mol Imaging. 2019;46:1787–95.
Acknowledgments
This work was supported by the São Paulo Research Foundation (FAPESP) in Brazil, reference number 2012/50239-6. The authors thank the staff of the Departments of Neurology and Psychiatry of the University of Sao Paulo Medical School for the selection and referral of the patients, and the staff of the Nuclear Medicine Center of the Institute of Radiology for the technical support.
Funding
This study was funded by the São Paulo Research Foundation (FAPESP) in Brazil, reference number 2012/50239-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Artur Martins Coutinho declares that he has no conflicts of interest.
Fábio Henrique de Gobbi Porto has received a speaker honorarium from Libbs, Lundbeck, and Sandoz-Novartis.
Daniele de Paula Faria declares that she has no conflicts of interest.
Carla Rachel Ono declares that she has no conflicts of interest.
Alexandre Teles Garcez declares that he has no conflicts of interest.
Paula Squarzoni declares that she has no conflicts of interest.
Fábio Luiz de Souza Duran declares that he has no conflicts of interest.
Maira Okada de Oliveira declares that she has no conflicts of interest.
Eduardo Sturzeneker Tres declares that he has no conflicts of interest.
Sonia Maria Dozzi Brucki declares that she has no conflicts of interest.
Orestes Vicente Forlenza declares that he has no conflicts of interest.
Ricardo Nitrini declares that he has no conflicts of interest.
Geraldo Busatto Filho declares that he has no conflicts of interest.
Carlos Alberto Buchpiguel declares that he has no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Coutinho, A.M., Busatto, G.F., de Gobbi Porto, F.H. et al. Brain PET amyloid and neurodegeneration biomarkers in the context of the 2018 NIA-AA research framework: an individual approach exploring clinical-biomarker mismatches and sociodemographic parameters. Eur J Nucl Med Mol Imaging 47, 2666–2680 (2020). https://doi.org/10.1007/s00259-020-04714-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04714-0