Abstract
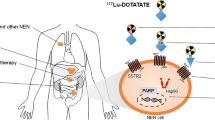
The incidence of neuroendocrine tumours (NETs) is increasing, but curative therapeutic options are limited because diagnosis is often delayed until the tumour has metastasized. Peptide receptor radionuclide therapy (PRRT) is among the most effective therapeutic options for metastatic NETs because of targeted delivery of radioactivity to the tumour via the somatostatin receptor (SSTR) and relatively low systemic toxicity. However, current PRRT regimes result in palliation rather than cure, and higher doses of PRRT that might achieve remission would also be too toxic to the patients. Therefore, there is a need to improve PRRT of NETs by combining it with other agents to achieve maximum benefits from the internal radiation therapy, while sparing non-target organs from radiation toxicity. Here we review various current and potential combination strategies to improve 177Lu-octreotate-based PRRT of NET, some of which could also apply to other radionuclide therapies. These strategies include co-administered drugs that improve delivery of the radiopharmaceutical via increased tumour perfusion or through increased SSTR density at tumour surface. Other combinations are aimed at enhancing the biological effects of the radiation-induced DNA damage in tumour cells or generating additional DNA damage burden to effectively increase the cytotoxicity of PRRT. We also propose an algorithm for stratifying NET patients to receive or not combination therapies with PRRT. Considering that PRRT and many of these combination agents are already used for treating patients with NET and other cancers, the proposed strategies to improve the efficacy of PRRT could be rapidly translated into the clinic.



Similar content being viewed by others
References
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system: World Health Organization; 2010.
Kidd M, Modlin IM, Bodei L, Drozdov I. Decoding the molecular and mutational ambiguities of gastroenteropancreatic neuroendocrine neoplasm pathobiology. Cell Mol Gastroenterol Hepatol. 2015;1:131–53. https://doi.org/10.1016/j.jcmgh.2014.12.008.
Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–97. https://doi.org/10.1002/cncr.29099.
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017. https://doi.org/10.1001/jamaoncol.2017.0589.
Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. https://doi.org/10.1200/JCO.2009.22.8510.
Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33. https://doi.org/10.1056/NEJMoa1316158.
Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol: Off J Eur Soc Med Oncol. 2013;24:152–60. https://doi.org/10.1093/annonc/mds276.
Wong MH, Chan DL, Lee A, Li BT, Lumba S, Clarke SJ, et al. Systematic review and meta-analysis on the role of chemotherapy in advanced and metastatic neuroendocrine tumor (NET). PLoS One. 2016;11:e0158140. https://doi.org/10.1371/journal.pone.0158140.
Lamarca A, Elliott E, Barriuso J, Backen A, McNamara MG, Hubner R, et al. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract, a systematic review and meta-analysis: a lost cause? Cancer Treat Rev. 2016;44:26–41. https://doi.org/10.1016/j.ctrv.2016.01.005.
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. https://doi.org/10.1056/NEJMoa1003825.
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. https://doi.org/10.1056/NEJMoa1009290.
Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31.
Werner RA, Weich A, Kircher M, Solnes LB, Javadi MS, Higuchi T, et al. The theranostic promise for neuroendocrine tumors in the late 2010s - where do we stand, where do we go? Theranostics. 2018;8:6088–100. https://doi.org/10.7150/thno.30357.
Zamora V, Cabanne A, Salanova R, Bestani C, Domenichini E, Marmissolle F, et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis. 2010;42:220–5. https://doi.org/10.1016/j.dld.2009.07.018.
Cambiaghi V, Vitali E, Morone D, Peverelli E, Spada A, Mantovani G, et al. Identification of human somatostatin receptor 2 domains involved in internalization and signaling in QGP-1 pancreatic neuroendocrine tumor cell line. Endocrine. 2017;56:146–57. https://doi.org/10.1007/s12020-016-1026-2.
Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008;286:230–7. https://doi.org/10.1016/j.mce.2008.02.002.
Zhao P, Canals M, Murphy JE, Klingler D, Eriksson EM, Pelayo JC, et al. Agonist-biased trafficking of somatostatin receptor 2A in enteric neurons. J Biol Chem. 2013;288:25689–700. https://doi.org/10.1074/jbc.M113.496414.
Roosterman D, Kempkes C, Cottrell GS, Padilla BE, Bunnett NW, Turck CW, et al. Endothelin-converting enzyme-1 degrades internalized somatostatin-14. Endocrinology. 2008;149:2200–7. https://doi.org/10.1210/en.2007-1628.
Koenig JA, Kaur R, Dodgeon I, Edwardson JM, Humphrey PP. Fates of endocytosed somatostatin sst2 receptors and associated agonists. Biochem J. 1998;336(Pt 2):291–8.
Kolby L, Wangberg B, Ahlman H, Tisell LE, Fjalling M, Forssell-Aronsson E, et al. Somatostatin receptor subtypes, octreotide scintigraphy, and clinical response to octreotide treatment in patients with neuroendocrine tumors. World J Surg. 1998;22:679–83.
Brabander T, Teunissen JJ, Van Eijck CH, Franssen GJ, Feelders RA, de Herder WW, et al. Peptide receptor radionuclide therapy of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2016;30:103–14. https://doi.org/10.1016/j.beem.2015.10.005.
Wang M, Caruano AL, Lewis MR, Meyer LA, VanderWaal RP, Anderson CJ. Subcellular localization of radiolabeled somatostatin analogues: implications for targeted radiotherapy of cancer. Cancer Res. 2003;63:6864–9.
Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with (177)Lu-Dotatate in the phase III NETTER-1 trial. J Clin Oncol. 2018;36:2578–84. https://doi.org/10.1200/JCO.2018.78.5865.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. https://doi.org/10.1056/NEJMoa1607427.
Kim S-J, Pak K, Koo P, Kwak J, Chang S. The efficacy of 177Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015:1–7. https://doi.org/10.1007/s00259-015-3155-x.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. https://doi.org/10.1200/JCO.2007.15.2553.
Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. https://doi.org/10.1007/s00259-014-2893-5.
Bergsma H, Lom KV, Konijnenberg M, Kam B, Teunissen J, Herder W, et al. Therapy-related hematological malignancies after peptide receptor radionuclide therapy with 177Lu-DOTA-Octreotate: incidence, course & predicting factors in patients with GEP-NETs. J Nucl Med: Off Publ, Soc Nucl Med. 2017. https://doi.org/10.2967/jnumed.117.189712.
Navalkissoor S, Grossman A. Targeted alpha particle therapy for neuroendocrine tumours: the next generation of peptide receptor radionuclide therapy. Neuroendocrinology. 2019;108:256–64. https://doi.org/10.1159/000494760.
Radojewski P, Dumont R, Marincek N, Brunner P, Macke HR, Muller-Brand J, et al. Towards tailored radiopeptide therapy. Eur J Nucl Med Mol Imaging. 2015;42:1231–7. https://doi.org/10.1007/s00259-015-3030-9.
Fani M, Nicolas GP, Wild D. Somatostatin receptor antagonists for imaging and therapy. J Nucl Med: Off Publ, Soc Nucl Med. 2017;58:61S–6S. https://doi.org/10.2967/jnumed.116.186783.
Del Prete M, Buteau FA, Arsenault F, Saighi N, Bouchard LO, Beaulieu A, et al. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: initial results from the P-PRRT trial. Eur J Nucl Med Mol Imaging. 2019;46:728–42. https://doi.org/10.1007/s00259-018-4209-7.
Sundlov A, Sjogreen-Gleisner K, Svensson J, Ljungberg M, Olsson T, Bernhardt P, et al. Individualised 177Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry. Eur J Nucl Med Mol Imaging. 2017. https://doi.org/10.1007/s00259-017-3678-4.
Bison SM, Haeck JC, Bol K, Koelewijn SJ, Groen HC, Melis M, et al. Optimization of combined temozolomide and peptide receptor radionuclide therapy (PRRT) in mice after multimodality molecular imaging studies. EJNMMI Res. 2015;5:62. https://doi.org/10.1186/s13550-015-0142-y.
D’Onofrio M, Cingarlini S, Ortolani S, Crosara S, DER R, Vallerio P, et al. Perfusion CT changes in liver metastases from pancreatic neuroendocrine tumors during everolimus treatment. Anticancer Res. 2017;37:1305–11. https://doi.org/10.21873/anticanres.11448.
Froidevaux S, Hintermann E, Torok M, Macke HR, Beglinger C, Eberle AN. Differential regulation of somatostatin receptor type 2 (sst 2) expression in AR4-2J tumor cells implanted into mice during octreotide treatment. Cancer Res. 1999;59:3652–7.
Haug AR, Rominger A, Mustafa M, Auernhammer C, Goke B, Schmidt GP, et al. Treatment with octreotide does not reduce tumor uptake of (68)Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med: Off Publ, Soc Nucl Med. 2011;52:1679–83. https://doi.org/10.2967/jnumed.111.089276.
Cherk MH, Kong G, Hicks RJ, Hofman MS. Changes in biodistribution on (68)Ga-DOTA-Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging. 2018;18:3. https://doi.org/10.1186/s40644-018-0136-x.
Bernhardt P, Oddstig J, Kolby L, Nilsson O, Ahlman H, Forssell-Aronsson E. Effects of treatment with (177)Lu-DOTA-Tyr(3)-octreotate on uptake of subsequent injection in carcinoid-bearing nude mice. Cancer Biother Radiopharm. 2007;22:644–53. https://doi.org/10.1089/cbr.2007.333.
Oddstig J, Bernhardt P, Lizana H, Nilsson O, Ahlman H, Kolby L, et al. Inhomogeneous activity distribution of 177Lu-DOTA0-Tyr3-octreotate and effects on somatostatin receptor expression in human carcinoid GOT1 tumors in nude mice. Tumour Biol. 2012;33:229–39. https://doi.org/10.1007/s13277-011-0268-0.
Dalmo J, Spetz J, Montelius M, Langen B, Arvidsson Y, Johansson H, et al. Priming increases the anti-tumor effect and therapeutic window of 177Lu-octreotate in nude mice bearing human small intestine neuroendocrine tumor GOT1. EJNMMI Res. 2017;7:6. https://doi.org/10.1186/s13550-016-0247-y.
Oddstig J, Bernhardt P, Nilsson O, Ahlman H, Forssell-Aronsson E. Radiation-induced up-regulation of somatostatin receptor expression in small cell lung cancer in vitro. Nucl Med Biol. 2006;33:841–6. https://doi.org/10.1016/j.nucmedbio.2006.07.010.
Oddstig J, Bernhardt P, Nilsson O, Ahlman H, Forssell-Aronsson E. Radiation induces up-regulation of somatostatin receptors 1, 2, and 5 in small cell lung cancer in vitro also at low absorbed doses. Cancer Biother Radiopharm. 2011;26:759–65. https://doi.org/10.1089/cbr.2010.0921.
Taelman VF, Radojewski P, Marincek N, Ben-Shlomo A, Grotzky A, Olariu CI, et al. Upregulation of key molecules for targeted imaging and therapy. J Nucl Med: Off Publ, Soc Nucl Med. 2016;57:1805–10. https://doi.org/10.2967/jnumed.115.165092.
Veenstra MJ, van Koetsveld PM, Dogan F, Farrell WE, Feelders RA, Lamberts SW, et al. Epidrug-induced upregulation of functional somatostatin type 2 receptors in human pancreatic neuroendocrine tumor cells. Oncotarget. 2016. https://doi.org/10.18632/oncotarget.9462.
Arvidsson Y, Johanson V, Pfragner R, Wangberg B, Nilsson O. Cytotoxic effects of valproic acid on neuroendocrine tumour cells. Neuroendocrinology. 2016;103:578–91. https://doi.org/10.1159/000441849.
Sun L, Qian Q, Sun G, Mackey LV, Fuselier JA, Coy DH, et al. Valproic acid induces NET cell growth arrest and enhances tumor suppression of the receptor-targeted peptide-drug conjugate via activating somatostatin receptor type II. J Drug Target. 2016;24:169–77. https://doi.org/10.3109/1061186X.2015.1066794.
Fueger BJ, Hamilton G, Raderer M, Pangerl T, Traub T, Angelberger P, et al. Effects of chemotherapeutic agents on expression of somatostatin receptors in pancreatic tumor cells. J Nucl Med: Off Publ, Soc Nucl Med. 2001;42:1856–62.
Nayak TK, Atcher RW, Prossnitz ER, Norenberg JP. Enhancement of somatostatin-receptor-targeted (177)Lu-[DOTA(0)-Tyr(3)]-octreotide therapy by gemcitabine pretreatment-mediated receptor uptake, up-regulation and cell cycle modulation. Nucl Med Biol. 2008;35:673–8. https://doi.org/10.1016/j.nucmedbio.2008.05.003.
van Essen M, Krenning EP, Kam BL, de Herder WW, van Aken MO, Kwekkeboom DJ. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35:743–8. https://doi.org/10.1007/s00259-007-0688-7.
Hubble D, Kong G, Michael M, Johnson V, Ramdave S, Hicks RJ. 177Lu-octreotate, alone or with radiosensitising chemotherapy, is safe in neuroendocrine tumour patients previously treated with high-activity 111In-octreotide. Eur J Nucl Med Mol Imaging. 2010;37:1869–75. https://doi.org/10.1007/s00259-010-1483-4.
Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:302–11. https://doi.org/10.1007/s00259-010-1631-x.
Kong G, Thompson M, Collins M, Herschtal A, Hofman MS, Johnston V, et al. Assessment of predictors of response and long-term survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging. 2014;41:1831–44. https://doi.org/10.1007/s00259-014-2788-5.
Kashyap R, Hofman MS, Michael M, Kong G, Akhurst T, Eu P, et al. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2015;42:176–85. https://doi.org/10.1007/s00259-014-2906-4.
Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27:561–9. https://doi.org/10.1089/cbr.2012.1276.
Kesavan M, Claringbold PG, Turner JH. Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology. 2014;99:108–17. https://doi.org/10.1159/000362558.
Claringbold PG, Turner JH. Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-octreotate-capecitabine-temozolomide radiopeptide chemotherapy. Neuroendocrinology. 2016;103:432–9. https://doi.org/10.1159/000434723.
Lewin J, Cullinane C, Akhurst T, Waldeck K, Watkins DN, Rao A, et al. Peptide receptor chemoradionuclide therapy in small cell carcinoma: from bench to bedside. Eur J Nucl Med Mol Imaging. 2015;42:25–32. https://doi.org/10.1007/s00259-014-2888-2.
Pool SE, Bison S, Koelewijn SJ, van der Graaf LM, Melis M, Krenning EP, et al. mTOR inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res. 2013;73:12–8. https://doi.org/10.1158/0008-5472.CAN-11-2089.
Bison SM, Pool SE, Koelewijn SJ, van der Graaf LM, Groen HC, Melis M, et al. Peptide receptor radionuclide therapy (PRRT) with [(177)Lu-DOTA(0),Tyr(3)]octreotate in combination with RAD001 treatment: further investigations on tumor metastasis and response in the rat pancreatic CA20948 tumor model. EJNMMI Res. 2014;4:21. https://doi.org/10.1186/s13550-014-0021-y.
Claringbold PG, Turner JH. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): a phase I study. Cancer Biother Radiopharm. 2015;30:261–9. https://doi.org/10.1089/cbr.2015.1876.
Elf AK, Bernhardt P, Hofving T, Arvidsson Y, Forssell-Aronsson E, Wangberg B, et al. NAMPT inhibitor GMX1778 enhances the efficacy of 177Lu-DOTATATE treatment of neuroendocrine tumors. J Nucl Med: Off Publ, Soc Nucl Med. 2016. https://doi.org/10.2967/jnumed.116.177584.
Nonnekens J, van Kranenburg M, Beerens CE, Suker M, Doukas M, van Eijck CH, et al. Potentiation of peptide receptor radionuclide therapy by the PARP inhibitor olaparib. Theranostics. 2016;6:1821–32. https://doi.org/10.7150/thno.15311.
Purohit NK, Shah RG, Adant S, Hoepfner M, Shah GM, Beauregard JM. Potentiation of (177)Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget. 2018;9:24693–706. https://doi.org/10.18632/oncotarget.25266.
Spetz J, Langen B, Rudqvist N, Parris TZ, Helou K, Nilsson O, et al. Hedgehog inhibitor sonidegib potentiates 177Lu-octreotate therapy of GOT1 human small intestine neuroendocrine tumors in nude mice. BMC Cancer. 2017;17:528. https://doi.org/10.1186/s12885-017-3524-x.
Jin XF, Auernhammer CJ, Ilhan H, Lindner S, Nolting S, Maurer J, et al. Combination of 5-fluorouracil with epigenetic modifiers induces radiosensitization, somatostatin receptor 2 expression and radioligand binding in neuroendocrine tumor cells in vitro. J Nucl Med: Off Publ, Soc Nucl Med. 2019. https://doi.org/10.2967/jnumed.118.224048.
Cavalcanti E, Ignazzi A, De Michele F, Caruso ML. PDGFRalpha expression as a novel therapeutic marker in well-differentiated neuroendocrine tumors. Cancer Biol Ther. 2019;20:423–30. https://doi.org/10.1080/15384047.2018.1529114.
Yazdani S, Kasajima A, Tamaki K, Nakamura Y, Fujishima F, Ohtsuka H, et al. Angiogenesis and vascular maturation in neuroendocrine tumors. Hum Pathol. 2014;45:866–74. https://doi.org/10.1016/j.humpath.2013.09.024.
Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–18. https://doi.org/10.1200/JCO.2012.46.3653.
Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017. https://doi.org/10.1007/s10456-017-9562-9.
Stepien K, Ostrowski RP, Matyja E. Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med Oncol. 2016;33:101. https://doi.org/10.1007/s12032-016-0814-0.
Kleibeuker EA, Ten Hooven MA, Verheul HM, Slotman BJ, Thijssen VL. Combining radiotherapy with sunitinib: lessons (to be) learned. Angiogenesis. 2015;18:385–95. https://doi.org/10.1007/s10456-015-9476-3.
Villaume K, Blanc M, Gouysse G, Walter T, Couderc C, Nejjari M, et al. VEGF secretion by neuroendocrine tumor cells is inhibited by octreotide and by inhibitors of the PI3K/AKT/mTOR pathway. Neuroendocrinology. 2010;91:268–78. https://doi.org/10.1159/000289569.
Kratochwil C, Stefanova M, Mavriopoulou E, Holland-Letz T, Dimitrakopoulou-Strauss A, Afshar-Oromieh A, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol: MIB: Off Publ Acad Mol Imaging. 2015;17:313–8. https://doi.org/10.1007/s11307-014-0795-3.
Ilan E, Sandstrom M, Wassberg C, Sundin A, Garske-Roman U, Eriksson B, et al. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med: Off Publ, Soc Nucl Med. 2015;56:177–82. https://doi.org/10.2967/jnumed.114.148437.
Slama A, Videau C, Kordon C, Epelbaum J. Estradiol regulation of somatostatin receptors in the arcuate nucleus of the female rat. Neuroendocrinology. 1992;56:240–5.
Vidal C, Rauly I, Zeggari M, Delesque N, Esteve JP, Saint-Laurent N, et al. Up-regulation of somatostatin receptors by epidermal growth factor and gastrin in pancreatic cancer cells. Mol Pharmacol. 1994;46:97–104.
Riaz H, Dong P, Shahzad M, Yang L. Constitutive and follicle-stimulating hormone-induced action of somatostatin receptor-2 on regulation of apoptosis and steroidogenesis in bovine granulosa cells. J Steroid Biochem Mol Biol. 2014;141:150–9. https://doi.org/10.1016/j.jsbmb.2014.02.001.
Nelson LE, Sheridan MA. Insulin and growth hormone stimulate somatostatin receptor (SSTR) expression by inducing transcription of SSTR mRNAs and by upregulating cell surface SSTRs. Am J Physiol Regul Integr Comp Physiol. 2006;291:R163–9. https://doi.org/10.1152/ajpregu.00754.2005.
Kimura N, Takamatsu N, Yaoita Y, Osamura RY, Kimura N. Identification of transcriptional regulatory elements in the human somatostatin receptor sst2 promoter and regions including estrogen response element half-site for estrogen activation. J Mol Endocrinol. 2008;40:75–91. https://doi.org/10.1677/JME-07-0108.
Pscherer A, Dorflinger U, Kirfel J, Gawlas K, Ruschoff J, Buettner R, et al. The helix-loop-helix transcription factor SEF-2 regulates the activity of a novel initiator element in the promoter of the human somatostatin receptor II gene. EMBO J. 1996;15:6680–90.
Xu Y, Berelowitz M, Bruno JF. Characterization of the promoter region of the human somatostatin receptor subtype 2 gene and localization of sequences required for estrogen-responsiveness. Mol Cell Endocrinol. 1998;139:71–7.
Zimmermann N, Lazar-Karsten P, Keck T, Billmann F, Schmid S, Brabant G, et al. Expression pattern of CDX2, estrogen and progesterone receptors in primary gastroenteropancreatic neuroendocrine tumors and metastases. Anticancer Res. 2016;36:921–4.
Sica G, Wagner PL, Altorki N, Port J, Lee PC, Vazquez MF, et al. Immunohistochemical expression of estrogen and progesterone receptors in primary pulmonary neuroendocrine tumors. Arch Pathol Lab Med. 2008;132:1889–95. https://doi.org/10.1043/1543-2165-132.12.1889.
Presky DH, Schonbrunn A. Somatostatin pretreatment increases the number of somatostatin receptors in GH4C1 pituitary cells and does not reduce cellular responsiveness to somatostatin. J Biol Chem. 1988;263:714–21.
Liu Z, Marquez M, Nilsson S, Holmberg AR. Incubation with somatostatin, 5-aza decitabine and trichostatin up-regulates somatostatin receptor expression in prostate cancer cells. Oncol Rep. 2008;20:151–4.
Degirmenci M, Erdogan AP, Bulut G, Atmaca H, Uzunoglu S, Karaca B, et al. Octreotide in combination with AT-101 induces cytotoxicity and apoptosis through up-regulation of somatostatin receptors 2 and 5 in DU-145 prostate cancer cells. Tumour Biol. 2016;37:4939–44. https://doi.org/10.1007/s13277-015-4331-0.
Basu S, Ostwal V. Observation on enhanced avidity on somatostatin receptor targeted 68Ga-DOTATATE PET-CT following therapy with everolimus and capecitabine-temozolamide: is redifferentiation akin phenomenon a reality in neuroendocrine tumors? Nucl Med Commun. 2016;37:669–71. https://doi.org/10.1097/MNM.0000000000000507.
Thakral P, Sen I, Pant V, Gupta SK, Dureja S, Kumari J, et al. Dosimetric analysis of patients with gastro entero pancreatic neuroendocrine tumors (NETs) treated with PRCRT (peptide receptor chemo radionuclide therapy) using Lu-177 DOTATATE and capecitabine/temozolomide (CAP/TEM). Br J Radiol. 2018;91:20170172. https://doi.org/10.1259/bjr.20170172.
Fan X, Mao Z, He D, Liao C, Jiang X, Lei N, et al. Expression of somatostatin receptor subtype 2 in growth hormone-secreting pituitary adenoma and the regulation of miR-185. J Endocrinol Investig. 2015;38:1117–28. https://doi.org/10.1007/s40618-015-0306-7.
Melis M, Forrer F, Capello A, Bijster M, Bernard BF, Reubi JC, et al. Up-regulation of somatostatin receptor density on rat CA20948 tumors escaped from low dose [177Lu-DOTA0,Tyr3]octreotate therapy. Q J Nucl Med Mol Imaging. 2007;51:324–33.
Behe MKS, Pqsken M, Gross M, Alfke H, Keil B, et al. Irradiation-induced upregulation of somatostatin and gastrin receptors in vitro and in vivo. Eur J Nucl Med Mol Imaging. 2004;31:S237–8.
Capello A, Krenning E, Bernard B, Reubi JC, Breeman W, de Jong M. 111In-labelled somatostatin analogues in a rat tumour model: somatostatin receptor status and effects of peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2005;32:1288–95. https://doi.org/10.1007/s00259-005-1877-x.
Driessen CM, de Boer JP, Gelderblom H, Rasch CR, de Jong MA, Verbist BM, et al. Induction chemotherapy with docetaxel/cisplatin/5-fluorouracil followed by randomization to two cisplatin-based concomitant chemoradiotherapy schedules in patients with locally advanced head and neck cancer (CONDOR study) (Dutch Head and Neck Society 08-01): a randomized phase II study. Eur J Cancer. 2016;52:77–84. https://doi.org/10.1016/j.ejca.2015.09.024.
Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol. 2004;22:2214–32. https://doi.org/10.1200/JCO.2004.08.009.
Kong G, Johnston V, Ramdave S, Lau E, Rischin D, Hicks RJ. High-administered activity In-111 octreotide therapy with concomitant radiosensitizing 5FU chemotherapy for treatment of neuroendocrine tumors: preliminary experience. Cancer Biother Radiopharm. 2009;24:527–33. https://doi.org/10.1089/cbr.2009.0644.
Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–21. https://doi.org/10.1038/nrm.2017.53.
Gupte R, Liu Z, Kraus WL. PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev. 2017;31:101–26. https://doi.org/10.1101/gad.291518.116.
Watson M, Roulston A, Belec L, Billot X, Marcellus R, Bedard D, et al. The small molecule GMX1778 is a potent inhibitor of NAD+ biosynthesis: strategy for enhanced therapy in nicotinic acid phosphoribosyltransferase 1-deficient tumors. Mol Cell Biol. 2009;29:5872–88. https://doi.org/10.1128/MCB.00112-09.
Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD metabolism in cancer therapeutics. Front Oncol. 2018;8:622. https://doi.org/10.3389/fonc.2018.00622.
Shah GM, Shah RG, Veillette H, Kirkland JB, Pasieka JL, Warner RR. Biochemical assessment of niacin deficiency among carcinoid cancer patients. Am J Gastroenterol. 2005;100:2307–14. https://doi.org/10.1111/j.1572-0241.2005.00268.x.
LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20:e15–28. https://doi.org/10.1016/S1470-2045(18)30786-1.
Hovstadius P, Larsson R, Jonsson E, Skov T, Kissmeyer AM, Krasilnikoff K, et al. A phase I study of CHS 828 in patients with solid tumor malignancy. Clin Cancer Res. 2002;8:2843–50.
Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, et al. PRRT genomic signature in blood for prediction of (177)Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging. 2018;45:1155–69. https://doi.org/10.1007/s00259-018-3967-6.
Acknowledgments
We would like to thank Marine A. Merlin for helpful discussion to improve the manuscript.
Funding
This work was supported by research funding to J.M.B. and G.M.S. from the Canadian Cancer Society Research Institute (grant no. 705327) and the Carcinoid-NeuroEndocrine Tumor Society of Canada,; and to J.M.B. from the Education and Research Foundation for Nuclear Medicine and Molecular Imaging, Quebec Bio-Imaging Network, Fondation du CHU de Québec – Université Laval and Fonds de Recherche du Québec – Santé. S.A. received a scholarship from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest related to this work. J.M.B. has received honoraria for invited conferences from Ipsen, Novartis, and Siemens Healthineers.
Ethical approval
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors other than those previously published and cited in this review article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – General
Rights and permissions
About this article
Cite this article
Adant, S., Shah, G.M. & Beauregard, JM. Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging 47, 907–921 (2020). https://doi.org/10.1007/s00259-019-04499-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04499-x