Abstract
Purpose
Reported outcomes of patients with intra-hepatic cholangiocarcinoma (IH-CCA) treated with radioembolization are highly variable, which indicates differences in included patients’ characteristics and/or procedure-related variables. This study aimed to identify patient- and treatment-related variables predictive for radioembolization outcome.
Methods
This retrospective multicenter study enrolled 58 patients with unresectable and chemorefractory IH-CCA treated with resin 90Y-microspheres. Clinicopathologic data were collected from patient records. Metabolic parameters of liver tumor(s) and presence of lymph node metastasis were measured on baseline 18F-FDG-PET/CT. 99mTc-MAA tumor to liver uptake ratio (TLRMAA) was computed for each lesion on the SPECT-CT. Activity prescription using body-surface-area (BSA) or more personalized partition-model was recorded. The study endpoint was overall survival (OS) starting from date of radioembolization. Statistical analysis was performed by the log-rank test and multivariate Cox’s proportional hazards model.
Results
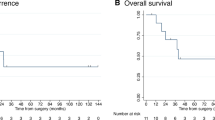
Median OS (mOS) post-radioembolization of the entire cohort was 10.3 months. Variables associated with significant differences in terms of OS were serum albumin (hazard ratio (HR) = 2.78, 95%CI:1.29–5.98, p = 0.002), total bilirubin (HR = 2.17, 95%CI:1.14–4.12, p = 0.009), aspartate aminotransferase (HR = 2.96, 95%CI:1.50–5.84, p < 0.001), alanine aminotransferase (HR = 2.02, 95%CI:1.05–3.90, p = 0.01) and γ-GT (HR = 2.61, 95%CI:1.31–5.22, p < 0.001). The presence of lymph node metastasis as well as a TLRMAA < 1.9 were associated with shorter mOS: HR = 2.35, 95%CI:1.08–5.11, p = 0.008 and HR = 2.92, 95%CI:1.01–8.44, p = 0.009, respectively. Finally, mOS was significantly shorter in patients treated according to the BSA method compared to the partition-model: mOS of 5.5 vs 14.9 months (HR = 2.52, 95%CI:1.23–5.16, p < 0.001). Multivariate analysis indicated that the only variable that increased outcome prediction above the clinical variables was the activity prescription method with HR of 2.26 (95%CI:1.09–4.70, p = 0.03). The average mean radiation dose to tumors was significantly higher with the partition-model (86Gy) versus BSA (38Gy).
Conclusion
Radioembolization efficacy in patients with unresectable recurrent and/or chemorefractory IH-CCA strongly depends on the tumor radiation dose. Personalized activity prescription should be performed.


Similar content being viewed by others
References
Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. Hpb. 2008;10:77–82. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1365182X1530023X. Accessed Apr 2008.
Cucchetti A, Cappelli A, Mosconi C, Zhong JH, Cescon M, Pinna AD, et al. Improving patient selection for selective internal radiation therapy of intra-hepatic cholangiocarcinoma: a meta-regression study. Liver Int. 2017;37:1056–64.
Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver. 2009;3:298–305.
Tan JCC, Coburn NG, Baxter NN, Kiss A, Law CHL. Surgical management of intrahepatic cholangiocarcinoma—a population-based study. Ann Surg Oncol. 2008;15:600–8.
Currie B, Soulen M. Decision making: intra-arterial therapies for cholangiocarcinoma—TACE and TARE. Semin Intervent Radiol. 2017;34:092–100. https://doi.org/10.1055/s-0037-1602591.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. https://doi.org/10.1056/NEJMoa0908721.
Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36:440–8.
Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–91. https://doi.org/10.1245/s10434-009-0777-x.
Hoffmann RT, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr EM, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–16.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2755245&tool=pmcentrez&rendertype=abstract. Accessed 1 Oct 2009.
Grosser OS, Ulrich G, Furth C, Pech M, Ricke J, Amthauer H, et al. Intrahepatic activity distribution in radioembolization with Yttrium-90–labeled resin microspheres using the body surface area method—a less than perfect model. J Vasc Interv Radiol. 2015;26(11):1615–21. Available from: https://www.sciencedirect.com/science/article/pii/S1051044315006995?via%3Dihub. Accessed 28 Aug 2015.
Ho S, Lau WY, Leung TWT, Chan M, Ngar YK, Johnson PJ, et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med. 1996;23:947–52.
Dieudonne A., Garin E, Laffont S, Rolland Y, Lebtahi R, Leguludec D, et al. Clinical feasibility of fast 3-dimensional dosimetry of the liver for treatment planning of hepatocellular carcinoma with 90Y-Microspheres. J Nucl Med 2011;52:1930–1937.
Levillain H, Duran Derijckere I, Marin G, Guiot T, Vouche M, Reynaert N, et al. 90Y-PET/CT-based dosimetry after selective internal radiation therapy predicts outcome in patients with liver metastases from colorectal cancer. EJNMMI Res. 2018;8:60. https://doi.org/10.1186/s13550-018-0419-z.
van den Hoven AF, Rosenbaum CENM, Elias SG, de Jong HWAM, Koopman M, Verkooijen HM, et al. Insights into the dose-response relationship of radioembolization with resin 90Y-Microspheres: a prospective cohort study in patients with colorectal cancer liver metastases. J Nucl Med. 2016;57:1014–9. https://doi.org/10.2967/jnumed.115.166942.
Willowson KP, Hayes AR, Chan DLH, Tapner M, Bernard EJ, Maher R, et al. Clinical and imaging-based prognostic factors in radioembolisation of liver metastases from colorectal cancer: a retrospective exploratory analysis. EJNMMI Res. 2017;7:46. https://doi.org/10.1186/s13550-017-0292-1.
Yttrium S-S. Resin microspheres [package insert]. North Sydney. Australia: SirTex Medical; 2017. Available from: https://www.sirtex.com/media/155126/ssl-us-13.pdf.
Wasan HS, Gibbs P, Sharma NK, Taieb J, Heinemann V, Ricke J, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017;18:1159–71.
Gibbs P, Gebski V, Van Buskirk M, Thurston K, Cade DN, Van Hazel GA, et al. Selective internal radiation therapy (SIRT) with yttrium-90 resin microspheres plus standard systemic chemotherapy regimen of FOLFOX versus FOLFOX alone as first-line treatment of non-resectable liver metastases from colorectal cancer: the SIRFLOX study. BMC Cancer. 2014;14:1–10.
Dutton SJ, Kenealy N, Love SB, Wasan HS, Sharma RA. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional selective internal radiation therapy (SIRT) as first-line treatment for patients with unresectable liver-on. BMC Cancer. 2014;14:497. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/279/CN-01115279/frame.html. Accessed 31 Jan 2016.
Cremonesi M, Chiesa C, Strigari L, Ferrari M, Botta F, Guerriero F, et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front Oncol. 2014;4:210.
Kao YH, Hock Tan AE, Burgmans MC, Irani FG, Khoo LS, Gong Lo RH, et al. Image-guided personalized predictive dosimetry by artery-specific SPECT/CT partition modeling for safe and effective 90Y Radioembolization. J Nucl Med 2012;53:559–566. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22343503. Accessed 17 Sep 2012.
Woff E, Hendlisz A, Garcia C, Deleporte A, Delaunoit T, Maréchal R, et al. Monitoring metabolic response using FDG PET-CT during targeted therapy for metastatic colorectal cancer. Eur J Nucl Med Mol Imaging. 2016;43:1792–801. https://doi.org/10.1007/s00259-016-3365-x.
Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48–54.
Haug AR, Heinemann V, Bruns CJ, Hoffmann R, Jakobs T, Bartenstein P, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2011;38:1037–45.
Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gener R, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105:1123–32.
Flamen P, Vanderlinden B, Delatte P, Ghanem G, Ameye L, Van Den Eynde M, et al. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with Yttrium-90 labeled resin microspheres. Phys Med Biol. 2008;53:6591–603.
Garin E, Lenoir L, Rolland Y, Edeline J, Mesbah H, Laffont S, et al. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass Microspheres: preliminary results. J Nucl Med. 2012;53:255–63. https://doi.org/10.2967/jnumed.111.094235.
Manceau V, Palard X, Rolland Y, Pracht M, Le Sourd S, Laffont S, et al. A MAA-based dosimetric study in patients with intrahepatic cholangiocarcinoma treated with a combination of chemotherapy and 90Y-loaded glass microsphere selective internal radiation therapy. Eur J Nucl Med Mol Imaging. 2018;45(10):1731–41. Available from: https://link.springer.com/article/10.1007%2Fs00259-018-3990-7. Accessed 20 Mar 2018.
Acknowledgments
This academic work was supported and sponsored by the Jules Bordet Institute. Part of the results was presented at the 2019 SNMMI–annual congress of the Society of Nuclear Medicine and Molecular Imaging as an oral presentation during the GI – Colorectal, liver, esophageal session (OP- 216).
Funding
This work was not supported by a grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PF, AH, HA and CD played an advisory role and received honoraria from Sirtex.
ML is a consultant for BTG, Sirtex, Quirem and Terumo. He receives research support from BTG, Quirem and Terumo. The department of Radiology and Nuclear Medicine of the UMC Utrecht receives royalties from Quirem.
BV played an advisory role and received honoraria from Dosisoft.
The first author and all other co-authors have no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Jules Bordet Institute Ethics Committee (CE2575) and Ethics Committees of all other participating centres. For this type of study formal consent is not required.
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Digestive Tract.
Electronic supplementary material
Supplementary material 1
18F-FDG-PET/CT and 99mTc-SPECT/CT patient based analysis (XLSX 14 kb)
Supplementary material 2
Correlation matrix of the 20 continuous variables (PDF 332 kb)
Supplementary material 3
Comparison between patients treated with the BSA method and patients treated with the partition-model (XLSX 16 kb)
Rights and permissions
About this article
Cite this article
Levillain, H., Duran Derijckere, I., Ameye, L. et al. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: a multicenter study. Eur J Nucl Med Mol Imaging 46, 2270–2279 (2019). https://doi.org/10.1007/s00259-019-04427-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04427-z