Abstract
Purpose
18F-fluoroaminosuberic acid (18F-FASu) is a recently developed amino acid tracer for positron emission tomography (PET) of oxidative stress that may offer improved tumour assessment over the conventional tracer 18F-fluorodeoxyglucose (18F-FDG). Our aim was to evaluate and relate dynamic 18F-FASu and 18F-FDG uptake with pharmacokinetic modelling to transporter protein expression levels in a panel of diverse tumour xenograft lines.
Methods
Four different tumour xenograft lines were implanted in female athymic nude mice: MAS98.12 and HBCx3 (breast), TPMX (osteosarcoma) and A549 (lung). Dynamic PET over 60 min was performed on a small animal unit. The time–activity curves (TACs) for 18F-FASu and 18F-FDG in individual tumours were used to extract early (SUVE; 2 min p.i.) and late (SUVL; 55 min p.i.) standardised uptake values. Pharmacokinetic two-tissue compartment models were applied to the TACs to estimate rate constants K1–k4 and blood volume fraction vB. Relative levels of cystine/glutamate antiporter subunit xCT were assessed by western blotting, and expression of GLUT1 and CD31 by immunohistochemistry.
Results
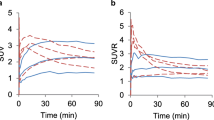
18F-FASu showed higher SUVE, whilst 18F-FDG exhibited higher SUVL. Influx rate K1 for 18F-FASu was significantly correlated with xCT levels (p = 0.001) and was significantly higher than K1 for 18F-FDG (p < 0.001). K1 for 18F-FDG was significantly correlated with GLUT1 levels (p = 0.002). vB estimated from 18F-FASu and 18F-FDG TACs was highly consistent and significantly correlated (r = 0.85, p < 0.001). Two qualitatively different 18F-FASu uptake profiles were identified: type α with low xCT expression and low K1 (A549 and HBCx3), and type β with high xCT expression and high K1 (MAS98.12 and TPMX).
Conclusion
The influx rate of 18F-FASu reflects xCT activity in tumour xenografts. Dynamic PET with pharmacokinetic modelling is needed to fully appraise 18F-FASu distribution routes.



Similar content being viewed by others
References
Inoue T, Kim EE, Komaki R, Wong FCL, Bassa P, Wong W-H, et al. Detecting recurrent or residual lung cancer with FDG-PET. J Nucl Med. 1995;36:788–93.
Hamada K, Tomita Y, Inoue A, Fujimoto T, Hashimoto N, Myoui A, et al. Evaluation of chemotherapy response in osteosarcoma with FDG-PET. Ann Nucl Med. 2009;23:89–95. https://doi.org/10.1007/s12149-008-0213-5.
McDermott GM, Welch A, Staff RT, Gilbert FJ, Schweiger L, Semple SI, et al. Monitoring primary breast cancer throughout chemotherapy using FDG-PET. Breast Cancer Res Treat. 2007;102:75–84.
Gulyas B, Halldin C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q J Nucl Med Mol Imaging. 2012;56:173–90.
Salminen E, Hogg A, Binns D, Frydenberg M, Hicks R. Investigations with FDG-PET scanning in prostate Cancer show limited value for clinical practice. Acta Oncol. 2002;41:425–9. https://doi.org/10.1080/028418602320405005.
Rosenbaum SJ, Lind T, Antoch G, Bockisch A. False-positive FDG PET uptake−the role of PET/CT. Eur Radiol. 2006;16:1054–65. https://doi.org/10.1007/s00330-005-0088-y.
Baek S, Choi C-M, Ahn SH, Lee JW, Gong G, Ryu J-S, et al. Exploratory clinical trial of (4S)-4-(3-[18F]fluoropropyl)-l-glutamate for imaging xC− transporter using positron emission tomography in patients with non–small cell lung or breast Cancer. Clin Cancer Res. 2012;18:5427–37. https://doi.org/10.1158/1078-0432.ccr-12-0214.
Baek S, Mueller A, Lim Y-S, Lee HC, Lee Y-J, Gong G, et al. (4S)-4-(3-18F-fluoropropyl)-l-glutamate for imaging of xC transporter activity in hepatocellular carcinoma using PET: preclinical and exploratory clinical studies. J Nucl Med. 2013;54:117–23.
Webster JM, Morton CA, Johnson BF, Yang H, Rishel MJ, Lee BD, et al. Functional imaging of oxidative stress with a novel PET imaging agent, 18F-5-fluoro-L-aminosuberic acid. J Nucl Med. 2014;55:657–64.
Yang H, Miao Q, Jenni S, Čolović M, Johnson B, Rishel M, et al. [18F] 5-fluoro aminosuberic acid (FASu) for oxidative stress imaging in breast cancer. J Nucl Med. 2015;56:1176.
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12:376–90. https://doi.org/10.1016/j.arr.2012.10.004.
Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009;11:2701–16. https://doi.org/10.1089/ars.2009.2692.
Belotte J, Fletcher NM, Awonuga AO, Alexis M, Abu-Soud HM, Saed MG, et al. The role of oxidative stress in the development of cisplatin resistance in epithelial ovarian cancer. Reprod Sci. 2014;21:503–8. https://doi.org/10.1177/1933719113503403.
Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–8.
Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x c − : cystine supplier and beyond. Amino Acids. 2012;42:231–46. https://doi.org/10.1007/s00726-011-0867-5.
Koeppe RA, Frey KA, Vander Borght TM, Karlamangla A, Jewett DM, Lee LC, et al. Kinetic evaluation of [11C]Dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab. 1996;16:1288–99. https://doi.org/10.1097/00004647-199611000-00025.
Kristian A, Nilsen LB, Røe K, Revheim M-E, Engebråten O, Mælandsmo GM, et al. Dynamic 18 F-FDG PET for assessment of tumor physiology in two breast carcinoma xenografts. Nucl Med Mol Imaging. 2013;47:173–80.
Kristian A, Riss P, Qu H, Milde M, Schoultz BW, Engebraaten O, et al. Positron emission tomography and pharmacokinetics of 2-[18F]-fluoroethyl choline for metabolic studies in breast cancer xenografts. Acta Oncol. 2014;53:1086–92. https://doi.org/10.3109/0284186X.2014.934398.
Bruheim S, Bruland OS, Breistol K, Maelandsmo GM, Fodstad Ø. Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol Oncol Res. 2004;10:133–41. https://doi.org/10.1007/bf03033741.
Pitman KE, Rusten E, Kristian A, Malinen E. Variability of dynamic 18F-FDG-PET data in breast cancer xenografts. Acta Oncol. 2015;54:1399–407.
Røe K, Aleksandersen TB, Kristian A, Nilsen LB, Seierstad T, Qu H, et al. Preclinical dynamic 18F-FDG PET-tumor characterization and radiotherapy response assessment by kinetic compartment analysis. Acta Oncol. 2010;49:914–21.
Turkheimer FE, Hinz R, Cunningham VJ. On the undecidability among kinetic models: from model selection to model averaging. J Cereb Blood Flow Metab. 2003;23:490–8.
Bretschi M, Cheng C, Witt H, Dimitrakopoulou-Strauss A, Strauss LG, Semmler W, et al. Cilengitide affects tumor compartment, vascularization and microenvironment in experimental bone metastases as shown by longitudinal 18F-FDG PET and gene expression analysis. J Cancer Res Clin Oncol. 2013;139:573–83. https://doi.org/10.1007/s00432-012-1360-6.
S-l S, Deng C, L-f W, J-j L, Wang H, Feng D, et al. 18F-FDG PET/CT-related metabolic parameters and their value in early prediction of chemotherapy response in a VX2 tumor model. Nucl Med Biol. 2009;37:327–33. https://doi.org/10.1016/j.nucmedbio.2009.12.002.
Wertheimer E, Sasson S, Cerasi E, Ben-Neriah Y. The ubiquitous glucose transporter GLUT-1 belongs to the glucose-regulated protein family of stress-inducible proteins. Proc Natl Acad Sci U S A. 1991;88:2525–9. https://doi.org/10.1073/pnas.88.6.2525.
Koppula P, Zhang Y, Shi J, Li W, Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J Biol Chem 2017:jbc. M117. 798405.
Čolović M, Rousseau E, Zhang Z, Lau J, Zhang C, Kuo H-T, et al. Synthesis and evaluation of an 18F-labeled boramino acid analog of aminosuberic acid for PET imaging of the antiporter system xC−. Bioorg Med Chem Lett. 2018;28:3579–84.
Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2007;27:1618. https://doi.org/10.1038/sj.onc.1210796 https://www.nature.com/articles/1210796#supplementary-information.
Osborne DR, Acuff S. Whole-body dynamic imaging with continuous bed motion PET/CT. Nucl Med Commun. 2016;37:428–31. https://doi.org/10.1097/MNM.0000000000000455.
Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. https://doi.org/10.1177/1534735404270335.
Funding
This study was funded by the University of Oslo (convergence grants) and the Norwegian Cancer Society (grant 6871141-2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence)
Rights and permissions
About this article
Cite this article
Pitman, K.E., Alluri, S.R., Kristian, A. et al. Influx rate of 18F-fluoroaminosuberic acid reflects cystine/glutamate antiporter expression in tumour xenografts. Eur J Nucl Med Mol Imaging 46, 2190–2198 (2019). https://doi.org/10.1007/s00259-019-04375-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04375-8