Abstract
Purpose
Huntington’s disease (HD) is a fatal neurodegenerative disorder with no effective treatment currently available. Although the pathological hallmark of HD is massive striatal atrophy, it has been suggested that cortical deterioration may concomitantly occur and play a major role in the patient’s functional independence. Our objective was to characterize cortical structural and metabolic neurodegeneration in the transition from premanifest to early-stage Huntington’s disease (HD).
Methods
Using a surface-based neuroimaging approach, we compared cortical thickness and intracortical FDG-PET uptake in 19 early-symptomatic HD patients with respect to 21 premanifest HD individuals.
Results
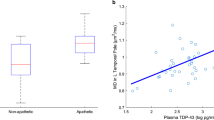
Early-HD patients showed significant cortical atrophy and intracortical hypometabolism when compared to premanifest subjects (p < 0.05, corrected for multiple comparisons). However, whereas the atrophy pattern was restricted to precentral and parieto-occipital regions, a pronounced frontotemporal hypometabolism was observed. Importantly, structural changes correlated with motor and cognitive performance, and metabolic changes were associated with the presence and severity of apathy in this population, a core neuropsychiatric feature of this disorder.
Conclusion
Our findings reveal an asynchronous neuronal loss and metabolic compromise across the cerebral cortex in early HD. Hence, the use of structural and metabolic imaging indicators to characterize disease progression in this population should take into consideration the dissociation which occurs between cortical atrophy and hypometabolism.

Similar content being viewed by others
References
Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98.
Nopoulos PC, Aylward EH, Ross CA, Johnson HJ, Magnotta VA, Juhl AR, et al. Cerebral cortex structure in prodromal Huntington disease. Neurobiol Dis. 2010;40:544–54.
Scahill RI, Andre R, Tabrizi SJ, Aylward EH. Structural imaging in premanifest and manifest Huntington disease. Handb Clin Neurol. 2017;144:247–61.
Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791–801.
Iwamoto T, Utsumi K, Kobayashi S, Yasumura S, Hatakeyama S, Hayashi A, et al. Effect of memantine on brain metabolic activity and perfusion in drug-naïve moderate Alzheimer’s disease patients. Neuropsychiatry. 2018;08:546–54.
Small GW, Siddarth P, Silverman DHS, Ercoli LM, Miller KJ, Lavretsky H, et al. Cognitive and cerebral metabolic effects of celecoxib versus placebo in people with age-related memory loss: randomized controlled study. Am J Geriatr Psychiatry. 2008;16:999–1009.
Hjermind LE, Law I, Jønch A, Stokholm J, Nielsen JE. Huntington’s disease: effect of memantine on FDG-PET brain metabolism? J Neuropsychiatr Clin Neurosci. 2011;23:206–10.
Pagano G, Niccolini F, Politis M. Current status of PET imaging in Huntington’s disease. Eur J Nucl Med Mol Imaging. 2016;43:1171–82.
Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, et al. Different partial volume correction methods lead to different conclusions: an (18)F-FDG-PET study of aging. NeuroImage. 2016;132:334–43.
Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. NeuroImage. 2014;92:225–36.
Martinez-Horta S, Perez-Perez J, van Duijn E, Fernandez-Bobadilla R, Carceller M, Pagonabarraga J, et al. Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s disease. Parkinsonism Relat Disord. 2016;25:58–64.
Kingma EM, van Duijn E, Timman R, van der Mast RC, Roos RAC. Behavioural problems in Huntington’s disease using the problem behaviours assessment. Gen Hosp Psychiatry. 2008;30:155–61.
Martínez-Horta S, Perez-Perez J, Sampedro F, Pagonabarraga J, Horta-Barba A, Carceller-Sindreu M, et al. Structural and metabolic brain correlates of apathy in Huntington’s disease. Mov Disord. 2018;33:1151–9.
Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5.
Lopez-Mora DA, et al. Selection of reference regions to model neurodegeneration in Huntington’s disease by FDG-PET/CT using imaging and clinical parameters. Clin Nucl Med. 2018;44(1):e1–e5.
Labuschagne I, Cassidy AM, Scahill RI, Johnson EB, Rees E, O’Regan A, et al. Visuospatial processing deficits linked to posterior brain regions in premanifest and early stage Huntington’s disease. J Int Neuropsychol Soc. 2016;22:595–608.
Johnson EB, Rees EM, Labuschagne I, Durr A, Leavitt BR, Roos RAC, et al. The impact of occipital lobe cortical thickness on cognitive task performance: an investigation in Huntington’s disease. Neuropsychologia. 2015;79:138–46.
Starkstein SE, Brandt J, Bylsma F, Peyser C, Folstein M, Folstein SE. Neuropsychological correlates of brain atrophy in Huntington’s disease: a magnetic resonance imaging study. Neuroradiology. 1992;34:487–9.
Coppen EM, van der Grond J, Hafkemeijer A, Barkey Wolf JJH, Roos RAC. Structural and functional changes of the visual cortex in early Huntington’s disease. Hum Brain Mapp. 2018;39(12):4776–86.
Arney K. Improved metrics for Huntington’s disease trials. Nature. 2018;557:S46–7.
Tabrizi SJ, Reilmann R, Roos RAC, Durr A, Leavitt B, Owen G, et al. Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 2012;11:42–53.
López-Mora DA, Camacho V, Pérez-Pérez J, Martínez-Horta S, Fernández A, Sampedro F, et al. Striatal hypometabolism in premanifest and manifest Huntington’s disease patients. Eur J Nucl Med Mol Imaging. 2016;43:2183–9.
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701.
Acknowledgements
The authors wish to thank all those at the Hospital de la Santa Creu i Sant Pau involved in the study. The authors also wish to thank the study participants and their families.
Funding
This study was partially funded by a Spanish Government Grant (PI17/001885).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Frederic Sampedro declares that he has no conflict of interest. Author Saul Martínez-Horta declares that he has no conflict of interest. Author Jesus Perez-Perez declares that he has no conflict of interest. Author Andrea Horta-Barba declares that she has no conflict of interest. Author Diego-Alfonso Lopez-Mora declares that he has no conflict of interest. Author Valle Camacho declares that she has no conflict of interest. Author Alejandro Fernandez-Leon declares that he has no conflict of interest. Author Beatriz Gomez-Anson declares that she has no conflict of interest. Author Ignasi Carrió declares that he has no conflict of interest. Author Jaime Kulisevsky declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sampedro, F., Martínez-Horta, S., Perez-Perez, J. et al. Cortical atrophic-hypometabolic dissociation in the transition from premanifest to early-stage Huntington’s disease. Eur J Nucl Med Mol Imaging 46, 1111–1116 (2019). https://doi.org/10.1007/s00259-018-4257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4257-z