Abstract
Purpose
The endoplasmic reticulum (ER) contains hexose-6P-dehydrogenase (H6PD). This enzyme competes with glucose-6P-phosphatase for processing a variety of phosphorylated hexoses including 2DG-6P. The present study aimed to verify whether this ER glucose-processing machinery contributes to brain FDG uptake.
Methods
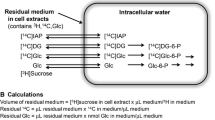
Effect of the H6PD inhibitor metformin on brain 18F-FDG accumulation was studied, in vivo, by microPET imaging. These data were complemented with the in vitro estimation of the lumped constant (LC). Finally, reticular accumulation of the fluorescent 2DG analogue 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2NBDG) and its response to metformin was studied by confocal microscopy in cultured neurons and astrocytes.
Results
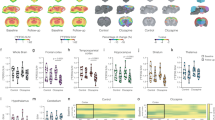
Metformin halved brain 18F-FDG accumulation without altering whole body tracer clearance. Ex vivo, this same response faced the doubling of both glucose consumption and lactate release. The consequent fall in LC was not explained by any change in expression or activity of its theoretical determinants (GLUTs, hexokinases, glucose-6P-phosphatase), while it agreed with the drug-induced inhibition of H6PD function. In vitro, 2NBDG accumulation selectively involved the ER lumen and correlated with H6PD activity being higher in neurons than in astrocytes, despite a lower glucose consumption.
Conclusions
The activity of the reticular enzyme H6PD profoundly contributes to brain 18F-FDG uptake. These data challenge the current dogma linking 2DG/FDG uptake to the glycolytic rate and introduce a new model to explain the link between 18-FDG uptake and neuronal activity.







Similar content being viewed by others
References
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The 14C-deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916.
Varrone A, Asenbaum S, Vander-Borght T, Booij J, Nobili F, Någren K, et al. EANM procedure guidelines for PET brain imaging using [18F]-FDG. Eur J Nucl Med Mol Imaging. 2009;36:2103–10.
Sols A. Substrate specificity of brain hexokinase. J Biol Chem. 1954;210:581–95.
Bachelard HS. Specificity and kinetic properties of monosaccharide uptake into Guinea pig cerebral cortex in vitro. J Neurochem. 1971;18:213–22.
Dienel GA, Cruz NF, Sokoloff L, Driscoll BF. Determination of glucose utilization rates in cultured astrocytes and neurons with [14C] deoxyglucose: progress, pitfalls, and discovery of intracellular glucose compartmentation. Neurochem Res. 2017;42:50–63.
Kanazawa Y, Yamane H, Shinohara S, Kuribayashi S, Momozono Y, Yamato Y, et al. 2-Deoxy-2-Fluoro-D-glucose as a functional probe for NMR: the unique metabolism beyond its 6-phosphate. J Neurochem. 1996;66:2113–20.
Dienel GA, Cruz NF. Synthesis of deoxyglucose-1-phosphate, deoxyglucose1,6-biphosphate, and other metabolites of 2-deoxy-D-[14C]glucose in rat brain in vivo: influence of time and tissue glucose level. J Neurochem. 1993;60:2217–31.
Southworth R, Parry CR, Parkes HG, Medina RA, Garlick PB. Tissue-specific differences in 2-fluoro-2-deoxyglucose metabolism beyond FDG-6-P: a 19 F NMR spectroscopy study in the rat. NMR Biomed. 2003;16(8):494–502.
Marini C, Ravera S, Buschiazzo A, Bianchi G, Orengo AM, Bruno S, et al. Discovery of a novel glucose metabolism in cancer: the role of endoplasmic reticulum beyond glycolysis and pentose phosphate shunt. Sci Rep. 2016;6:25092.
Senesi S, Csala M, Marcolongo P, Fulceri R, Mandl J, Banhegyi G, et al. Hexose-6-phosphate dehydrogenase in the endoplasmic reticulum. Biol Chem. 2010;391:1–8.
Kulkarnit P, Hodgson E. Mouse liver microsomal hexose-6-phosphate dehydrogenase. Biochem Pharmacol. 1982;31:1131–7.
Moreira PI. Metformin in the diabetic brain: friend or foe? Ann Transl Med. 2014;2:2–4.
Correia S, Carvalho C, Santos MS, Proença T, Nunes E, Duarte AI, et al. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008;4:358–64.
Blumrich EM, Dringen R. Metformin accelerates glycolytic lactate production in cultured primary cerebellar granule neurons. Neurochem Res. 2017:1–12. https://doi.org/10.1007/s11064-017-2346-1.
Westhaus A, Maria E, Dringen R. The antidiabetic drug metformin stimulates glycolytic lactate production in cultured primary rat astrocytes. Neurochem Res. 2015;123:1–16.
Buschiazzo A, Cossu V, Bauckneht M, Orengo A, Piccioli P, Emionite L, et al. Effect of starvation on brain glucose metabolism and 18F-2-fluoro-2- deoxyglucose uptake: an experimental in-vivo and ex-vivo study. EJNMMI Res. 2018. https://doi.org/10.1186/s13550-018-0398-0.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7.
Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, et al. The 18F-fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res. 1979;44(1):127–37.
Graham MM, Muzi M, Spence AM, O'Sullivan F, Lewellen TK, Link JM, et al. The FDG lumped constant in normal human brain. J Nucl Med. 2002;43:1157–67.
Noll T, Mühlensiepen H, Engels R, Hamacher K, Papaspyrou M, Langen KJ, et al. A cell-culture reactor for the on-line evaluation of radiopharmaceuticals: evaluation of the lumped constant of FDG in human glioma cells. J Nucl Med. 2000;41:556–64.
Suda S, Shinohara M, Miyaoka M, Lucignani G, Kennedy C, Sokoloff L. The lumped constant of the deoxyglucose method in hypoglycemia: effects of moderate hypoglycemia on local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1990;10:499–509.
Blomqvist G, Seitz RJ, Sjögren I, Halldin C, Stone-Elander S, Widén L, et al. Regional cerebral oxidative and total glucose consumption during rest and activation studied with positron emission tomography. Acta Physiol Scand. 1994;151:29–43.
Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003.
Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400.
Cura AJ, Carruthers A. Role of monosaccharide transport proteins in carbohydrate assimilation , distribution, metabolism , and homeostasis. Compr Physiol. 2012;2:863–914.
Guionie O, Clottes E, Stafford K, Burchell A. Identification and characterisation of a new human glucose-6-phosphatase isoform. FEBS Lett. 2003;551:159–64.
Muzi M, Freeman SD, Burrows RC, Wiseman RW, Link JM, Krohn KA, et al. Kinetic characterization of hexokinase isoenzymes from glioma cells: implications for FDG imaging of human brain tumors. Nucl Med Biol. 2001;28:107–16.
Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 2005;64:207–15.
Sokoloff L. Relation between physiological function and energy metabolism in the central nervous system. J Neurochem. 1977;29:13–26.
Van Schaftingen E, Gerin I. The glucose-6-phosphatase system. Biochem J. 2002;532:513–32.
Caracó C, Aloj L, Chen LY, Chou JY, Eckelman WC. Cellular release of [18F]2-Fluoro-2-deoxyglucose as a function of the glucose-6-phosphatase enzyme system. J Biol Chem. 2000;275:18489–94.
Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Neurobiology. 1994;91:10625–9.
Dienel GA. Review lack of appropriate stoichiometry: strong evidence against an energetically important astrocyte–neuron lactate shuttle in brain. J Neurosci Res. 2017;95:2103–25.
Acknowledgements
The authors are indebted with Proff. Antonio De Flora, Alessandro Morelli and Alberto Pupi for the enthusiastic support and criticisms.
Funding
This study was funded by the program “Ricerca Corrente,” line “Guest-Cancer Interactions,” by Compagnia di San Paolo (project ID Prot.: 2015.AAI4110.U4917).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures involving animals were performed in respect of the current National and International regulations and were reviewed and approved by the Licensing and Animal Welfare Body of the IRCCS Ospedale Policlinico San Martino, Genoa, Italy and by the Italian Ministry of Health.
Conflict of interests
No author has any conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cossu, V., Marini, C., Piccioli, P. et al. Obligatory role of endoplasmic reticulum in brain FDG uptake. Eur J Nucl Med Mol Imaging 46, 1184–1196 (2019). https://doi.org/10.1007/s00259-018-4254-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4254-2