Abstract
Purpose
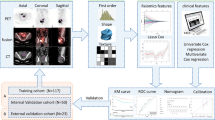
The aim of this study was to validate previously developed radiomics models relying on just two radiomics features from 18F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) images for prediction of disease free survival (DFS) and locoregional control (LRC) in locally advanced cervical cancer (LACC).
Methods
Patients with LACC receiving chemoradiotherapy were enrolled in two French and one Canadian center. Pre-treatment imaging was performed for each patient. Multicentric harmonization of the two radiomics features was performed with the ComBat method. The models for DFS (using the feature from apparent diffusion coefficient (ADC) MRI) and LRC (adding one PET feature to the DFS model) were tuned using one of the French cohorts (n = 112) and applied to the other French (n = 50) and the Canadian (n = 28) external validation cohorts.
Results
The DFS model reached an accuracy of 90% (95% CI [79–98%]) (sensitivity 92–93%, specificity 87–89%) in both the French and the Canadian cohorts. The LRC model reached an accuracy of 98% (95% CI [90–99%]) (sensitivity 86%, specificity 100%) in the French cohort and 96% (95% CI [80–99%]) (sensitivity 83%, specificity 100%) in the Canadian cohort. Accuracy was significantly lower without ComBat harmonization (82–85% and 71–86% for DFS and LRC, respectively). The best prediction using standard clinical variables was 56–60% only.
Conclusions
The previously developed PET/MRI radiomics predictive models were successfully validated in two independent external cohorts. A proposed flowchart for improved management of patients based on these models should now be confirmed in future larger prospective studies.







Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Moore KN, Java JJ, Slaughter KN, Rose PG, Lanciano R, DiSilvestro PA, et al. Is age a prognostic biomarker for survival among women with locally advanced cervical cancer treated with chemoradiation? An NRG oncology/gynecologic oncology group ancillary data analysis. Gynecol Oncol. 2016;143(2):294–301.
Herrera FG, Prior JO. The role of PET/CT in cervical cancer. Front Oncol. 2013;3:34.
Choi J, Kim HJ, Jeong YH, Lee JH, Cho A, Yun M, et al. The role of (18) F-FDG PET/CT in assessing therapy response in cervix cancer after concurrent chemoradiation therapy. Nucl Med Mol Imaging. 2014;48(2):130–6.
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–6.
Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol. 2016;61(13):R150–66.
Ho KC, Fang YH, Chung HW, Yen TC, Ho TY, Chou HH, et al. A preliminary investigation into textural features of intratumoral metabolic heterogeneity in (18)F-FDG PET for overall survival prognosis in patients with bulky cervical cancer treated with definitive concurrent chemoradiotherapy. Am J Nucl Med Mol Imaging. 2016;6(3):166–75.
Torheim T, Groendahl AR, Andersen EK, Lyng H, Malinen E, Kvaal K, et al. Cluster analysis of dynamic contrast enhanced MRI reveals tumor subregions related to locoregional relapse for cervical cancer patients. Acta Oncol. 2016;55(11):1294–8.
Chung HH, Kang SY, Ha S, Kim JW, Park NH, Song YS, et al. Prognostic value of preoperative intratumoral FDG uptake heterogeneity in early stage uterine cervical cancer. J Gynecol Oncol. 2016;27(2):e15.
Guan Y, Li W, Jiang Z, Chen Y, Liu S, He J, et al. Whole-lesion apparent diffusion coefficient-based entropy-related parameters for characterizing cervical cancers: initial findings. Acad Radiol. 2016;23(12):1559–67.
Reuze S, Orlhac F, Chargari C, Nioche C, Limkin E, Riet F, et al. Prediction of cervical cancer recurrence using textural features extracted from 18F-FDG PET images acquired with different scanners. Oncotarget. 2017;8(26):43169–79.
Lucia F, Visvikis D, Desseroit MC, Miranda O, Malhaire JP, Robin P, et al. Prediction of outcome using pretreatment (18)F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2018;45(5):768–86.
Zwanenburg A, Lock S. Why validation of prognostic models matters? Radiother Oncol J Eur Soc Ther Radiol Oncol. 2018;127(3):370–3.
Vallieres M, Zwanenburg A, Badic B, Cheze Le Rest C, Visvikis D, Hatt M. Responsible radiomics research for faster clinical translation. J Nucl Med. 2018;59(2):189–93.
Lim K, Small W Jr, Portelance L, Creutzberg C, Jurgenliemk-Schulz IM, Mundt A, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79(2):348–55.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Zwanenburg A, Leger S, Vallières M, Löck S. Image biomarker standardisation initiative - feature definitions. 2017.
Desseroit MC, et al. Comparison of three quantization methods for the calculation of textural features in PET/CT images: impact on prognostic models in non-small cell lung cancer. IEEE Nucl Sci Symp Med Imaging Conf 2016. 2016.
Chalkidou A, O'Doherty MJ, Marsden PK. False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One. 2015;10(5):e0124165.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur J Clin Investig. 2015;45(2):204–14.
Yang F, Dogan N, Stoyanova R, Ford JC. Evaluation of radiomic texture feature error due to MRI acquisition and reconstruction: a simulation study utilizing ground truth. Phys Med Int J Devoted Appl Phys Med Biol Off J Ital Assoc Biomed Phys. 2018;50:26–36.
Yan J, Chu-Shern JL, Loi HY, Khor LK, Sinha AK, Quek ST, et al. Impact of image reconstruction settings on texture features in 18F-FDG PET. J Nucl Med. 2015;56(11):1667–73.
Hatt M, Cheze le Rest C, Turzo A, Roux C, Visvikis D. A fuzzy locally adaptive Bayesian segmentation approach for volume determination in PET. IEEE Trans Med Imaging. 2009;28(6):881–93.
Hatt M, Cheze le Rest C, Descourt P, Dekker A, De Ruysscher D, Oellers M, et al. Accurate automatic delineation of heterogeneous functional volumes in positron emission tomography for oncology applications. Int J Radiat Oncol Biol Phys. 2010;77(1):301–8.
Velazquez ER, Parmar C, Jermoumi M, Mak RH, van Baardwijk A, Fennessy FM, et al. Volumetric CT-based segmentation of NSCLC using 3D-slicer. Sci Rep. 2013;3:3529.
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20.
Orlhac F, Boughdad S, Philippe C, Stalla-Bourdillon H, Nioche C, Champion L, Soussan M, Frouin F, Frouin V, Buvat I. A post-reconstruction harmonization method for multicenter radiomic studies in PET. J Nucl Med. 2018.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–27.
Fortin JP, Parker D, Tunc B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. NeuroImage. 2017;161:149–70.
Naik A, Gurjar OP, Gupta KL, Singh K, Nag P, Bhandari V. Comparison of dosimetric parameters and acute toxicity of intensity-modulated and three-dimensional radiotherapy in patients with cervix carcinoma: a randomized prospective study. Cancer Radiother J de la Societe Francaise de Radiother Oncol. 2016;20(5):370–6.
Lin G, Yang LY, Lin YC, Huang YT, Liu FY, Wang CC, Lu HY, Chiang HJ, Chen YR, Wu RC, et al. Prognostic model based on magnetic resonance imaging, whole-tumour apparent diffusion coefficient values and HPV genotyping for stage IB-IV cervical cancer patients following chemoradiotherapy. Eur Radiol. 2018.
Galavis PE, Hollensen C, Jallow N, Paliwal B, Jeraj R. Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol. 2010;49(7):1012–6.
Goh WWB, Wang W, Wong L. Why batch effects matter in omics data, and how to avoid them. Trends Biotechnol. 2017;35(6):498–507.
Kothari S, Phan JH, Stokes TH, Osunkoya AO, Young AN, Wang MD. Removing batch effects from histopathological images for enhanced cancer diagnosis. IEEE J Biomed Health Inf. 2014;18(3):765–72.
Mackin D, Fave X, Zhang L, Yang J, Jones AK, Ng CS, et al. Harmonizing the pixel size in retrospective computed tomography radiomics studies. PLoS One. 2017;12(9):e0178524.
Wu J, Aguilera T, Shultz D, Gudur M, Rubin DL, Loo BW Jr, et al. Early-stage non-small cell lung cancer: quantitative imaging characteristics of (18)F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology. 2016;281(1):270–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors François Lucia, Dimitris Visvikis, Martin Vallières, Marie-Charlotte Desseroit, Omar Miranda, Philippe Robin, Pietro Andrea Bonaffini, Joanne Alfieri, Ingrid Masson, Augustin Mervoyer, Caroline Reinhold, Olivier Pradier, Mathieu Hatt, Ulrike Schick declare that they have no conflict of interest.
No financial support was received for this work.
There are no potential conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Statement of translational relevance describing how the results might be applied to the future practice of cancer medicine
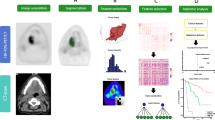
Our findings have a direct impact on patient management in clinical practice. A flowchart demonstrates how to exploit the two radiomics features necessary to guide and personalize treatment: the textural feature (EntropyGLCM) extracted from ADC maps derived from DWI-MRI acquisitions can identify patients with low risk of recurrence, for which it could be advised to avoid adjuvant treatment. Among the patients with a higher risk of recurrence, the second textural feature (GLNUGLRLM) extracted from the FDG PET can differentiate between patients with a risk of distant recurrence, for which a systemic adjuvant treatment or more intensive surveillance could be recommended, and those with locoregional relapse, for which a locoregional adjuvant treatment might be more beneficial. Both MRI and PET images are routinely acquired for LACC patients, and the radiomics features have standardized definition with the IBSI guidelines, therefore anyone could easily evaluate our models in their data.
Electronic supplementary material
ESM 1
(DOCX 442 kb)
Rights and permissions
About this article
Cite this article
Lucia, F., Visvikis, D., Vallières, M. et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging 46, 864–877 (2019). https://doi.org/10.1007/s00259-018-4231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4231-9