Abstract
Objectives
The purpose of this study was to compare the diagnostic value of a one-step to a two-step staging algorithm utilizing 18F-FDG PET/MRI in breast cancer patients.
Methods
A total of 38 patients (37 females and one male, mean age 57 ± 10 years; range 31–78 years) with newly diagnosed, histopathologically proven breast cancer were prospectively enrolled in this trial. All PET/MRI examinations were assessed for local tumor burden and metastatic spread in two separate reading sessions: (1) One-step algorithm comprising supine whole-body 18F-FDG PET/MRI, and (2) Two-step algorithm comprising a dedicated prone 18F-FDG breast PET/MRI and supine whole-body 18F-FDG PET/MRI.
Results
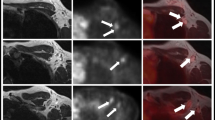
On a patient based analysis the two-step algorithm correctly identified 37 out of 38 patients with breast carcinoma (97%), while five patients were missed by the one-step 18F-FDG PET/MRI algorithm (33/38; 87% correct identification). On a lesion-based analysis 56 breast cancer lesions were detected in the two-step algorithm and 44 breast cancer lesions could be correctly identified in the one-step 18F-FDG PET/MRI (79%), resulting in statistically significant differences between the two algorithms (p = 0.0015). For axillary lymph node evaluation sensitivity, specificity and accuracy was 93%, 95 and 94%, respectively. Furthermore, distant metastases could be detected in seven patients in both algorithms.
Conclusion
The results demonstrate the necessity and superiority of a two-step 18F-FDG PET/MRI algorithm, comprising dedicated prone breast imaging and supine whole-body imaging, when compared to the one-step algorithm for local and whole-body staging in breast cancer patients.





Similar content being viewed by others
References
Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–37. https://doi.org/10.1002/cncr.29936.
Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. https://doi.org/10.1016/j.ejca.2009.12.014.
Michaelson JS, Chen LL, Silverstein MJ, Mihm MC Jr, Sober AJ, Tanabe KK, et al. How cancer at the primary site and in the lymph nodes contributes to the risk of cancer death. Cancer. 2009;115:5095–107. https://doi.org/10.1002/cncr.24592.
Riegger C, Herrmann J, Nagarajah J, Hecktor J, Kuemmel S, Otterbach F, et al. Whole-body FDG PET/CT is more accurate than conventional imaging for staging primary breast cancer patients. Eur J Nucl Med Mol Imaging. 2012;39:852–63. https://doi.org/10.1007/s00259-012-2077-0.
Heusner TA, Kuemmel S, Koeninger A, Hamami ME, Hahn S, Quinsten A, et al. Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging. 2010;37:1077–86. https://doi.org/10.1007/s00259-010-1399-z.
Heusner TA, Kuemmel S, Hahn S, Koeninger A, Otterbach F, Hamami ME, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging. 2009;36:1543–50. https://doi.org/10.1007/s00259-009-1145-6.
Garami Z, Hascsi Z, Varga J, Dinya T, Tanyi M, Garai I, et al. The value of 18-FDG PET/CT in early-stage breast cancer compared to traditional diagnostic modalities with an emphasis on changes in disease stage designation and treatment plan. Eur J Surg Oncol :J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2012;38:31–7. https://doi.org/10.1016/j.ejso.2011.09.002.
Filippi V, Malamitsi J, Vlachou F, Laspas F, Georgiou E, Prassopoulos V, et al. The impact of FDG-PET/CT on the management of breast cancer patients with elevated tumor markers and negative or equivocal conventional imaging modalities. Nucl Med Commun. 2011;32:85–90. https://doi.org/10.1097/MNM.0b013e328341c898.
Koolen BB, van der Leij F, Vogel WV, Rutgers EJ, Vrancken Peeters MJ, Elkhuizen PH, et al. Accuracy of 18F-FDG PET/CT for primary tumor visualization and staging in T1 breast cancer. Acta Oncol. 2014;53:50–7. https://doi.org/10.3109/0284186X.2013.783714.
Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. https://doi.org/10.1148/radiol.12110853.
Pinker K, Bogner W, Baltzer P, Gruber S, Bickel H, Brueck B, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Investig Radiol. 2014;49:421–30. https://doi.org/10.1097/RLI.0000000000000029.
Garcia-Velloso MJ, Ribelles MJ, Rodriguez M, Fernandez-Montero A, Sancho L, Prieto E, et al. MRI fused with prone FDG PET/CT improves the primary tumour staging of patients with breast cancer. Eur Radiol. 2017;27:3190–8. https://doi.org/10.1007/s00330-016-4685-8.
Sawicki LM, Grueneisen J, Schaarschmidt BM, Buchbender C, Nagarajah J, Umutlu L, et al. Evaluation of (1)(8)F-FDG PET/MRI, (1)(8)F-FDG PET/CT, MRI, and CT in whole-body staging of recurrent breast cancer. Eur J Radiol. 2016;85:459–65. https://doi.org/10.1016/j.ejrad.2015.12.010.
Janssen NNY, Ter Beek LC, Loo CE, Winter-Warnars G, Lange CAH, van Loveren M, et al. Supine breast MRI using respiratory triggering. Acad Radiol. 2017;24:818–25. https://doi.org/10.1016/j.acra.2017.01.003.
Peccatori FA, Codacci-Pisanelli G, Del Grande M, Scarfone G, Zugni F, Petralia G. Whole body MRI for systemic staging of breast cancer in pregnant women. Breast. 2017;35:177–81. https://doi.org/10.1016/j.breast.2017.07.014.
Pallone MJ, Poplack SP, Avutu HB, Paulsen KD, Barth RJ Jr. Supine breast MRI and 3D optical scanning: a novel approach to improve tumor localization for breast conserving surgery. Ann Surg Oncol. 2014;21:2203–8. https://doi.org/10.1245/s10434-014-3598-5.
Mallory MA, Sagara Y, Aydogan F, DeSantis S, Jayender J, Caragacianu D, et al. Feasibility of intraoperative breast MRI and the role of prone versus supine positioning in surgical planning for breast-conserving surgery. Breast J. 2017;23:713–7. https://doi.org/10.1111/tbj.12796.
Dietzel M, Zoubi R, Burmeister HP, Runnebaum IB, Kaiser WA, Baltzer PA. Combined staging at one stop using MR mammography: evaluation of an extended protocol to screen for distant metastasis in primary breast cancer - initial results and diagnostic accuracy in a prospective study. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2012;184:618–23. https://doi.org/10.1055/s-0031-1271117.
Oehmigen M, Lindemann ME, Lanz T, Kinner S, Quick HH. Integrated PET/MR breast cancer imaging: attenuation correction and implementation of a 16-channel RF coil. Med Phys. 2016;43:4808. https://doi.org/10.1118/1.4959546.
Quick HH. Integrated PET/MR. J Magn Reson Imaging: JMRI. 2014;39:243–58. https://doi.org/10.1002/jmri.24523.
Paulus DH, Quick HH. Hybrid positron emission tomography/magnetic resonance imaging: challenges, methods, and state of the art of hardware component attenuation correction. Investig Radiol. 2016;51:624–34. https://doi.org/10.1097/RLI.0000000000000289.
Kartmann R, Paulus DH, Braun H, Aklan B, Ziegler S, Navalpakkam BK, et al. Integrated PET/MR imaging: automatic attenuation correction of flexible RF coils. Med Phys. 2013;40:082301. https://doi.org/10.1118/1.4812685.
Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE. BI-RADS((R)) fifth edition: a summary of changes. Diagn Interv Imaging. 2017;98:179–90. https://doi.org/10.1016/j.diii.2017.01.001.
Moy L, Ponzo F, Noz ME, Maguire GQ Jr, Murphy-Walcott AD, Deans AE, et al. Improving specificity of breast MRI using prone PET and fused MRI and PET 3D volume datasets. J Nucl Med : Off Publ, Soc Nucl Med. 2007;48:528–37.
Heusner TA, Hahn S, Jonkmanns C, Kuemmel S, Otterbach F, Hamami ME, et al. Diagnostic accuracy of fused positron emission tomography/magnetic resonance mammography: initial results. Br J Radiol. 2011;84:126–35. https://doi.org/10.1259/bjr/93330765.
Grueneisen J, Nagarajah J, Buchbender C, Hoffmann O, Schaarschmidt BM, Poeppel T, et al. Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast Cancer: a comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Investig Radiol. 2015;50:505–13. https://doi.org/10.1097/RLI.0000000000000197.
Anderson BO, Distelhorst SR. Guidelines for international breast health and cancer control--implementation. Introduction Cancer. 2008;113:2215–6. https://doi.org/10.1002/cncr.23980.
Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol : Off J Am Soc Clin Oncol. 2008;26:3248–58. https://doi.org/10.1200/JCO.2007.15.2108.
Catalano OA, Daye D, Signore A, Iannace C, Vangel M, Luongo A, et al. Staging performance of whole-body DWI, PET/CT and PET/MRI in invasive ductal carcinoma of the breast. Int J Oncol. 2017;51:281–8. https://doi.org/10.3892/ijo.2017.4012.
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23:489–502. https://doi.org/10.1016/j.breast.2014.08.009.
Melsaether AN, Raad RA, Pujara AC, Ponzo FD, Pysarenko KM, Jhaveri K, et al. Comparison of whole-body (18)F FDG PET/MR imaging and whole-body (18)F FDG PET/CT in terms of lesion detection and radiation dose in patients with breast Cancer. Radiology. 2016;281:193–202. https://doi.org/10.1148/radiol.2016151155.
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma. J Nucl Med: Off Publ, Soc Nucl Med. 2012;53:1244–52. https://doi.org/10.2967/jnumed.112.109306.
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med: Off Publ, Soc Nucl Med. 2012;53:928–38. https://doi.org/10.2967/jnumed.112.105338.
Tabouret-Viaud C, Botsikas D, Delattre BM, Mainta I, Amzalag G, Rager O, et al. PET/MR in breast cancer. Semin Nucl Med. 2015;45:304–21. https://doi.org/10.1053/j.semnuclmed.2015.03.003.
Taneja S, Jena A, Goel R, Sarin R, Kaul S. Simultaneous whole-body (1)(8)F-FDG PET-MRI in primary staging of breast cancer: a pilot study. Eur J Radiol. 2014;83:2231–9. https://doi.org/10.1016/j.ejrad.2014.09.008.
Pace L, Nicolai E, Luongo A, Aiello M, Catalano OA, Soricelli A, et al. Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: lesion detection and quantitation of 18F-deoxyglucose uptake in lesions and in normal organ tissues. Eur J Radiol. 2014;83:289–96. https://doi.org/10.1016/j.ejrad.2013.11.002.
Gombos EC, Jayender J, Richman DM, Caragacianu DL, Mallory MA, Jolesz FA, et al. Intraoperative supine breast MR imaging to quantify tumor deformation and detection of residual breast cancer: preliminary results. Radiology. 2016;281:720–9. https://doi.org/10.1148/radiol.2016151472.
Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361–70. https://doi.org/10.1148/radiol.2016161444.
Heusch P, Buchbender C, Beiderwellen K, Nensa F, Hartung-Knemeyer V, Lauenstein TC, et al. Standardized uptake values for [(1)(8)F] FDG in normal organ tissues: comparison of whole-body PET/CT and PET/MRI. Eur J Radiol. 2013;82:870–6. https://doi.org/10.1016/j.ejrad.2013.01.008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kirchner, J., Grueneisen, J., Martin, O. et al. Local and whole-body staging in patients with primary breast cancer: a comparison of one-step to two-step staging utilizing 18F-FDG-PET/MRI. Eur J Nucl Med Mol Imaging 45, 2328–2337 (2018). https://doi.org/10.1007/s00259-018-4102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4102-4