Abstract
Background
Up-to-date information on human epidermal growth factor receptor 2 (HER2) status in breast cancer (BC) is important, as expression can vary during the course of the disease, necessitating anti-HER2 therapy adjustments. Repeat biopsies, however, are not always possible. In this feasibility trial we assessed whether 89Zr-trastuzumab PET could support diagnostic understanding and aid clinical decision making, when HER2 status could not be determined by standard work up. Additionally, HER2 status on circulating tumour cells (CTCs) was assessed.
Patients and methods
89Zr-trastuzumab PET was performed in patients if disease HER2 status remained unclear after standard work up (bone scan, 18F-FDG PET, CT and if feasible a biopsy). PET result and central pathologic revision of available tumour biopsies were reported to the referring physician. CTC HER2 status prior to PET was evaluated afterwards and therefore not reported. Diagnostic understanding and treatment decision questionnaires were completed by the referring physicians before, directly after and ≥ 3 months after 89Zr-trastuzumab PET.
Results
Twenty patients were enrolled: 8 with two primary cancers (HER2-positive and HER2-negative BC or BC and non-BC), 7 with metastases inaccessible for biopsy, 4 with prior HER2-positive and -negative metastases and 1 with primary BC with equivocal HER2 status. 89Zr-trastuzumab PET was positive in 12 patients, negative in 7 and equivocal in 1 patient. In 15/20 patients, 89Zr-trastuzumab PET supported treatment decision. The scan altered treatment of 8 patients, increased physicians’ confidence without affecting treatment in 10, and improved physicians’ disease understanding in 18 patients. In 10/20 patients CTCs were detected; 6/10 showed HER2 expression. CTC HER2 status was not correlated to 89Zr-trastuzumab PET result or treatment decision.
Conclusion
89Zr-trastuzumab PET supports clinical decision making when HER2 status cannot be determined by standard work up. The impact of CTC HER2 status needs to be further explored.
Similar content being viewed by others
Introduction
In metastatic breast cancer, treatment options are largely dependent upon the presence of the oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2), in addition to tumour load and location. The outcome of HER2 positive metastatic disease has fundamentally improved since the development of effective HER2 targeting agents such as trastuzumab, pertuzumab and trastuzumab-emtansine [1]. In this light, it is of particular interest that HER2 status can change during disease course, consequently necessitating anti-HER2 therapy adjustment. Furthermore, HER2 status discordancy between primary and residual or metastatic lesions, either HER2 loss or gain [2], was related to shorter disease-free and overall patient survival in retrospective [3, 4] and prospective analyses [5]. This discordancy, measured by immunohistochemistry (IHC) and/or in situ hybridization (ISH) techniques, ranged between 0 and 33% [2, 3, 6,7,8,9,10,11,12,13,14]. Moreover, HER2 expression can be heterogeneous within the same tumour [6, 15, 16]. Therefore, temporal and spatial heterogeneity may fundamentally affect HER2 status and therefore treatment response. Based on this data, clinical guidelines encourage repeat biopsies during the course of the disease. However, due to technical or patient related factors, tumour lesions are not always (safely) accessible, leaving the clinician with a dilemma with regard to the disease’s HER2 status.
HER2 imaging using 89Zr-trastuzumab positron emission tomography (PET) could be a strategy to noninvasively assess HER2 expression in tumour lesions throughout the whole body [17, 18]. It might, therefore, become a valuable tool to guide clinical decision making in metastatic breast cancer patients, who—despite extensive work-up—pose a clinical dilemma [19, 20]. Characterization of circulating tumour cells (CTCs) might be another patient-friendly method to assess HER2 status on metastatic cells [21]. Since CTCs are likely shed from different tumour sites—metastases and the primary tumour, if still present—they might reflect both HER2 status and tumour heterogeneity. Consequently, the aim of this clinical feasibility trial was to assess whether 89Zr-trastuzumab PET supports clinical decision making in patients suspected of metastatic or locally recurrent HER2-positive breast cancer, presenting with a dilemma defined as failure of the standard work-up to evaluate the present HER2 status of their disease. In addition, HER2 status of CTCs was assessed and correlated to treatment decision and 89Zr-trastuzumab PET result.
Patients and methods
Patient population
This prospective single-centre clinical trial protocol was approved by the medical ethics committee of the University Medical Centre Groningen (UMCG; ClinicalTrials.gov identifier NCT01832051). All patients provided written informed consent.
Patients with suspected metastatic disease or local recurrence of HER2-positive breast cancer with a clinical dilemma defined as failure of standard work-up to evaluate the HER2 status were eligible. HER2-positivity, reported in the patient’s history, was defined positive with an IHC of score 3+ or IHC of score 2+ followed by ISH showing HER2 amplification according to the American Society of Clinical Oncology guidelines [22]. Standard imaging work-up preferably consisted of a computed tomography (CT) of the chest and abdomen, a bone scintigraphy and a fluorine-18-fluorodeoxyglucose (18F-FDG) PET scan, accompanied by a metastasis biopsy, if feasible. Other eligibility criteria included age ≥ 18 years and Eastern Cooperative Oncology Group performance status of 0–2. Patients with a history of allergic reactions to immunoglobulins and pregnant or lactating women, as well as patients with any inabilities not allowing compliance with the study procedures, were excluded.
89Zr-trastuzumab PET scan
Clinical grade 89Zr-trastuzumab was produced at the UMCG as described previously [17]. Patients received 37 MBq (± 10%; ~1 m Ci) 89Zr-trastuzumab intravenously supplemented with unlabeled antibody to a total amount of 50 mg trastuzumab. Four days postinjection, head to upper thigh was scanned in up to nine bed positions with 5 min/bed position in combination with a low dose CT scan for attenuation correction and anatomic reference with a Biograph mCT 64-slice PET/CT camera (Siemens). PET scans were reconstructed and visually analysed by one dedicated nuclear medicine physician. The 89Zr-trastuzumab PET scan was considered positive, when in comparison to the 18F-FDG PET and in conjunction with conventional imaging (e.g. contrast enhanced CT scan, bone scan or MRI in case of brain metastases) the entire tumour load or a dominant part of the tumour load showed 89Zr-trastuzumab tumour uptake [23]. 89Zr-trastuzumab tumour uptake was considered substantial when tumour tracer uptake in visceral lesions (excluding brain) was at least comparable to or higher than liver background or in case of brain metastases when 89Zr-trastuzumab uptake was exceeding brain background uptake allowing clear identification of the metastasis. Interpretation of 89Zr-trastuzumab uptake in bone lesions was assessed in relation with visceral metastases.
Retrospectively, PET images were reconstructed using the harmonized reconstruction algorithm recommended for multicentre 89Zr-mAb PET scan trials [24] and all tumour lesions on the conventional imaging were recorded, including measurability according to RECIST 1.1 [25] and prior radiation therapy. Tumour lesions with a diameter of >15 mm on contrast enhanced CT scan were quantified, when tumour tracer uptake was considered not to be influenced by surrounding tissue and when a lesion was not irradiated ≤6 months of the 89Zr-trastuzumab PET scan. With the AMIDE (A Medical Image Data Examiner) software (version 0.9.3, [26]) radioactivity was quantified in manually drawn volumes of interest around tumour lesions and several background organs, and standardized uptake values (SUV) were calculated. We report SUVmax for tumour lesions and SUVmean for normal organ tracer uptake.
Clinical value
To assess the influence of the 89Zr-trastuzumab PET scan on treatment decision, referring physicians completed earlier validated questionnaires before, directly after and > 3 months after the 89Zr-trastuzumab PET scan [27]. Information on the patient’s history, which dilemma incited the referral for 89Zr-trastuzumab PET, as well as the intended treatment were assessed with the first questionnaire. In the second questionnaire, completed after receiving the scan result, the treating physician was asked to give the final diagnosis, the chosen treatment strategy and information on potential additional tests planned. With the last questionnaire, referring physicians were asked to rate the contribution of the 89Zr-trastuzumab PET scan to their diagnostic understanding of the patient’s disease and the choice of therapy using a 5-point scale (Supplementary Table S1). All questionnaires were checked for internal consistency.
Archival tumour samples
Available archival tumour samples from the primary tumour site(s) or metastases were centrally revised and IHC (SP3; rabbit monoclonal antibody; NeoMarkers, Lab Vision Corp., Thermo Fisher Scientific, Fremont, California, USA), and in case of an IHC 2+ score ISH (PathVysion HER2/neu DNA probe kit, Vysis, Abbott Molecular, Des Plaines, IL) were repeated. HER2 positivity was defined as IHC 3+, or IHC 2+ with a positive ISH (HER2:CEP17 ratio ≥ 2.0 or an average of ≥6.0 HER2 copies per nucleus; [22]).
Circulating tumour cell analysis
Before tracer injection, blood for CTC enumeration and CTC HER2 expression analysis was collected. Samples were transported to the laboratory of Clinical Tumour Immunology, Erasmus MC Cancer Institute, Rotterdam, the Netherlands, for analysis. One CellSave tube was used to obtain an EpCAM-based CTC count from 7.5 mL blood using the Epithelial Cell Kit (Janssen Diagnostics LLC, Raritan, NJ, USA) on CellSearch System according to the manufacturer’s instructions. CTCs were further characterized for HER2 expression within the Cell-Search system by a FITC-labelled anti-HER2 antibody as described by the manufacturer (CellSearch tumour phenotyping reagent HER2/neu; Janssen Diagnostics LLC). HER2 immunofluorescence staining intensity of 3+ and 2+ were scored as HER2-positive as described earlier [28]. CTC HER2 status was evaluated after inclusion of all patients and was not reported to the referring physician.
Statistical analysis
Statistical analyses were performed using SPSS Version 23. To assess relation between CTC result and 89Zr-trastuzumab scan result or chosen treatment strategy, Spearman’s correlation was used. P ≤ 0.05 was considered to be a significant difference. Data are presented as mean ± standard deviation (SD), unless otherwise stated.
Results
Patient characteristics
Twenty patients were enrolled between July 2013 and June 2015 from all over the Netherlands and the Northern border area of Germany, with a median distance to our centre of 125 km (range 20–247, Table 1). The 89Zr-trastuzumab PET scan was requested by the referring physicians (all: medical oncologists) due to following reasons (Supplementary Table S2): (i) To differentiate between metastases of two primary cancers, either two primary breast cancers (one HER2-positive and the other HER2-negative), or a HER2-positive breast cancer and a second primary cancer from another origin (N = 8), (ii) to assess HER2 status of a single lesion inaccessible for biopsy, or in case of multiple lesions inability to perform repeat biopsies (N = 7), (iii) to assess HER2 expression of metastatic breast cancer with known heterogeneous HER2 status over time (N = 4), and (iv) to evaluate HER2 expression in metastatic breast cancer with prior equivocal histopathological result (HER2 IHC score 2+, ISH result: average 4.23 HER2 gene copies/nucleus, N = 1).
89Zr-trastuzumab PET
The highest normal organ 89Zr-trastuzumab uptake was observed in the liver, followed by the kidney, intestine (=faeces), blood pool and the spleen; the lowest was seen in subcutaneous tissue and the brain (Supplementary Figure S1).
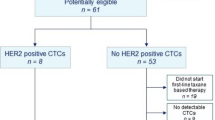
At visual assessment, 89Zr-trastuzumab tumour uptake was considered positive in 12 patients, negative in seven patients and equivocal in one patient (Fig. 1 and Supplementary Table S2).
Retrospectively, a total of 404 tumour lesions were delineated on 89Zr-trastuzumab PET after primary visual assessment of which 264 (65%) were considered evaluable. In two patients, none of the known metastases appeared on 89Zr-trastuzumab PET and their scans, therefore, were considered negative. In the remaining 18 patients a median of 9 lesions (range 1–69) was evaluable. Heterogeneity of tumour tracer uptake was observed within patients, with a maximal 8-fold difference within one patient. Also, tumour tracer uptake varied greatly between patients, with a maximal 13-fold difference (data not shown).
HER2 status in tumour biopsies in comparison to 89Zr-trastuzumab PET
For central revision a total of 42 tumour samples of 20 patients were available (primary N = 18, secondary N = 10, metastasis N = 14). One patient, who had reported HER2-positive disease, was diagnosed with heterogeneous disease after central pathology revision (Table 2). Furthermore, two out of ten patients with a reported combination of HER2-positive and HER2-negative disease and the one patient with the equivocal histopathological result were diagnosed with HER2-negative disease after central revision.
The 89Zr-trastuzumab PET scan was positive in seven out of eight patients with a previously HER2 positive primary tumour, and in five out of nine patients with a previous combination of HER2-positive and HER2-negative disease according to available tumour tissue (Table 2).
Clinical value of 89Zr-trastuzumab PET
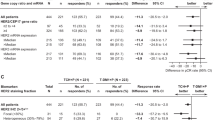
The work-up including 89Zr-trastuzumab PET scan improved the treating physician’s understanding of the patient’s disease in 18 (90%) patients (Fig. 2). The confidence over the (unaltered) treatment choice was improved in ten patients (50%), and in eight patients (40%) the treatment was changed. Five patients were started on anti-HER2 treatment and three patients did not receive HER2-targeting agents as a consequence of the 89Zr-trastuzumab scan (Table 3). In one patient the scan did not influence the understanding and/or treatment choice, and one physician of a patient with osteosarcoma and simultaneous HER2-positive breast cancer, rated choice of treatment based on the 89Zr-trastuzumab PET as non-beneficial for the patient, although the scan improved her understanding of the disease.
CTC HER2 status
CTCs were found in half of the patient population (median number of CTCs/7.5 mL = 6.5, range 1–99). In six of them, HER2-positive CTCs were found and three of the six patients also had a positive 89Zr-trastuzumab PET scan (Supplementary Table S3). Two out of the six patients, both with positive 89Zr-trastuzumab PET, received anti-HER2 treatment subsequently. Overall, CTC result was not correlated to 89Zr-trastuzumab PET result or subsequent treatment decision (correlation with PET result: r = 0.074, P = 0.84; correlation with treatment decision: r = −0.37, P = 0.92).
Discussion
In this small prospective clinical feasibility trial we show for the first time that 89Zr-trastuzumab PET can support diagnostic understanding and clinical decision making when HER2 status of metastatic or locally recurrent breast cancer cannot be determined by standard work up.
The 89Zr-trastuzumab PET scan improved the physician’s understanding of the patient’s disease in the majority of patients and the treatment strategy was changed in 40% of the study population. Five patients received initially unplanned anti-HER2 therapy, whereas in three patients, intended anti-HER2 therapy was withheld. By doing this, the latter patients were possibly saved from toxicity of a potentially ineffective treatment. Moreover, the savings of treatment-related costs outweigh scan-related costs manifold. Thereby, distance to 89Zr-trastuzumab PET was no issue in our trial as patients were willing to travel up to almost 250 km (~150 miles), implying that such molecular scan techniques, although localized only in specialized centres, can be within reach of a vast majority of patients. Using additional molecular imaging in standard clinical care will increase radiation exposure. In case of a 89Zr-trastuzumab PET, this additional radiation exposure equals that of one diagnostic CT scan of the chest, abdomen and pelvis [29,30,31,32]. The balance between risks and benefits of any additional procedure should always be carefully considered in any patient population. In this particular population, a diagnostic dilemma is known to negatively affect their survival if left unsolved. In light of the potentially helpful information gained by the scan and also considering the incurable nature of their disease, we think that the benefits of a 89Zr-trastuzumab PET outweigh the risks in this particular patient population. Therefore, we consider this scan as suitable for clinical practice.
CTC analysis in metastatic breast cancer has shown to be a strong prognostic factor [33,34,35,36]. Since CTCs probably originate from different tumour sites, they might also provide a comprehensive view of tumour characteristics like HER2 status, including tumour heterogeneity. In our trial, CTCs were only detected in half of the patients, which corresponds with the earlier reported CTC detection rate [36]. The impact of CTC HER2 status on clinical decision making is unclear from the present study, as the result was not reported to the referring physician. Therefore this will have to be further explored. However, central pathology revision including renewed HER2 staining, and subsequent comparison of primary tumour and metastases biopsies, did deliver new insights in HER2 status in three out of 20 patients in this study. Therefore this could be worth considering in the standard setting.
Validation of molecular scan techniques is still an ongoing process. Clinical utility of 89Zr-trastuzumab PET, especially the relation of scan results with treatment response and survival data in recently diagnosed metastatic breast cancer patients, is currently assessed in a prospective, multicentre observational cohort study conducted in the Netherlands (ClinicalTrials.gov Identifier: NCT01957332). In this trial, intra-patient heterogeneity of tumour tracer uptake will also further be evaluated, as so far the clinical implication of the observed heterogeneity is unclear. The trial, furthermore, supports validation and standardization of interpretation of this PET imaging technique, which is instrumental for potential further wider application as possible biomarker for treatment response in the future. Additionally, the impact of CTC enumeration and characterization for HER2 and its relation with 89Zr-trastuzumab PET is further explored in the mentioned trial. However, the present study already establishes 89Zr-trastuzumab PET as a diagnostic tool to help the treating physician in clinical decision making, in this niche population of patients with an otherwise undetermined HER2 status of their disease.
References
Thomas A, Khan S, Lynch C, et al. Survival by HER2 receptor status in stage IV breast cancer: SEER 2010–2012. J Clin Oncol. 2017;35(suppl; abstr 1032).
Lower EE, Glass E, Blau R, et al. HER2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat. 2009;113:301–6.
Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30:593–9.
Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–8.
Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–8.
Masood S, Bui MM. Assessment of HER2/neu overexpression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann Clin Lab Sci. 2000;30:259–65.
Gancberg D, Di Leo A, Cardoso F, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–43.
Regitnig P, Schippinger W, Lindbauer M, et al. Change of HER2/neu status in a subset of distant metastases from breast carcinomas. J Pathol. 2004;203:918–26.
Zidan J, Dashkovsky I, Stayerman C, et al. Comparison of HER2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–6.
Vincent-Salomon A, Jouve M, Genin P, et al. HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer. 2002;94:2169–73.
Tanner M, Jarvinen P, Isola J. Amplification of HER2/neu and topoisomerase IIα in primary and metastatic breast cancer. Cancer Res. 2001;61:5345–8.
Santinelli A, Pisa E, Stramazzotti D, et al. HER2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008;122:999–1004.
Gong Y, Booser DJ, Sneige N. Comparison of HER2 status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer. 2005;103:1763–9.
Curigliano G, Bagnardi V, Viale G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol. 2011;22:2227–33.
Wu JM, Halushka MK, Argani P. Intratumoral heterogeneity of HER-2 gene amplification and protein overexpression in breast cancer. Hum Pathol. 2010;41:914–7.
Hanna W, Nofech-Mozes S, Kahn HJ. Intratumoral heterogeneity of HER2/neu in breast cancer--a rare event. Breast J. 2007;13:122–9.
Dijkers EC, Kosterink JG, Rademaker AP, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009;50:974–81.
Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nucl Med. 2016;57:1523–8.
Gaykema SB, Brouwers AH, Hovenga S, et al. Zirconium-89-trastuzumab positron emission tomography as a tool to solve a clinical dilemma in a patient with breast cancer. J Clin Oncol. 2012;30:e74–5.
Bensch F, van Rooijen JM, Schroder CP, et al. A 21-year-old patient with a HER2-positive colorectal cancer. Gastroenterology. 2015;148:20–1.
Onstenk W, Gratama JW, Foekens JA, et al. Towards a personalized breast cancer treatment approach guided by circulating tumor cell (CTC) characteristics. Cancer Treat Rev. 2013;39:691–700.
Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27:619–24.
Makris NE, Boellaard R, Visser EP, et al. Multicenter harmonization of 89Zr PET/CT performance. J Nucl Med. 2014;55:264–7.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–7.
Herder GJ, Van Tinteren H, Comans EF, et al. Prospective use of serial questionnaires to evaluate the therapeutic efficacy of 18F-fluorodeoxyglucose positron emission tomography in suspected lung cancer. Thorax. 2003;58:47–51.
Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16:2634–45.
Borjesson PK, Jauw YW, de Bree R, et al. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J Nucl Med. 2009;50:1828–36.
Makris NE, Boellaard R, van Lingen A, et al. PET/CT-derived whole-body and bone marrow dosimetry of 89Zr-cetuximab. J Nucl Med. 2015;56:249–54.
McCollough CH, Bushberg JT, Fletcher JG, et al. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc. 2015;90:1380–92.
Eschner W, Schmidt M, Dietlein M, et al. PROLARA: prognosis-based lifetime attributable risk approximation for cancer from diagnostic radiation exposure. Eur J Nucl Med Mol Imaging. 2010;37:131–5.
Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging-predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9.
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Plaks V, Koopman CD, Werb Z. Cancer. Circulating tumor cells. Science. 2013;341:1186–8.
Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14.
Acknowledgements
We thank Linda Pot and Rianne Bakker for technical support for the labelling.
Funding
This work was financially supported by an unrestricted research grant from the Dutch A Sister’s Hope foundation provided to C. P. Schröder.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.G.E. de Vries received research support from Hoffmanm-La Roche and Genentech (payment to the institution). All other authors declared no competing interests.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary table S1
(DOCX 15 kb)
Supplementary table S2
(DOCX 23 kb)
Supplementary table S3
(DOCX 20 kb)
Supplementary figure S1.
Normal organ 89Zr-trastuzumab distribution depicted as mean SUVmean (+SD) (JPG 542 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bensch, F., Brouwers, A.H., Lub-de Hooge, M.N. et al. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging 45, 2300–2306 (2018). https://doi.org/10.1007/s00259-018-4099-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4099-8