Abstract
Purpose
Evaluation of response to immunotherapy is a matter of debate. The aim of the present study was to evaluate the response of metastatic melanoma to treatment with ipilimumab by means of 18F-FDG PET/CT, using the patients’ clinical response as reference.
Methods
The final cohort included in the analyses consisted of 41 patients with metastatic melanoma who underwent 18F-FDG PET/CT before and after administration of ipilimumab. After determination of the best clinical response, the PET/CT scans were reviewed and a separate independent analysis was performed, based on the number and functional size of newly emerged 18F-FDG-avid lesions, as well as on the SUV changes after therapy.
Results
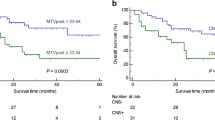
The median observation time of the patients after therapy was 21.4 months (range 6.3–41.9 months). Based on their clinical response, patients were dichotomized into those with clinical benefit (CB) and those without CB (No-CB). The CB group (31 patients) included those with stable disease, partial remission and complete remission, and the No-CB group (10 patients) included those with progressive disease. The application of a threshold of four newly emerged 18F-FDG-avid lesions on the posttherapy PET/CT scan led to a sensitivity (correctly predicting CB) of 84% and a specificity (correctly predicting No-CB) of 100%. This cut-off was lower for lesions with larger functional diameters (three new lesions larger than 1.0 cm and two new lesions larger than 1.5 cm). SUV changes after therapy did not correlate with clinical response. Based on these findings, we developed criteria for predicting clinical response to immunotherapy by means of 18F-FDG PET/CT (PET Response Evaluation Criteria for Immunotherapy, PERCIMT).
Conclusion
Our results show that a cut-off of four newly emerged 18F-FDG-avid lesions on posttherapy PET/CT gives a reliable indication of treatment failure in patients under ipilimumab treatment. Moreover, the functional size of the new lesions plays an important role in predicting the clinical response. Validation of these results in larger cohorts of patients is warranted.




Similar content being viewed by others
References
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82.
Missailidis S. Anticancer therapeutics. Chichester: Wiley-Blackwell; 2008.
Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2015;42:386–96.
Balch CM, Gershenwald JE, Soong S-J, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206.
Lin E, Alavi A. PET and PET/CT: a clinical guide. New York: Thieme; 2009.
PMOD Technologies. Iso-contour mode (Pseudo-Snake). Zürich: PMOD Technologies LLC. http://www.pmod.com/files/download/v31/doc/pbas/4729.htm. Accessed 5 November 2017.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52.
Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily – CTLA-4. Nature. 1987;328:267–70.
Food and Drug Administration. Yervoy® (ipilimumab) injection, for intravenous use. Silver Spring, MD: Food and Drug Administration; 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125377s073lbl.pdf. Accessed 5 November 2017.
Goode EF, Smyth EC. Immunotherapy for gastroesophageal cancer. J Clin Med. 2016;5, 84.
Dimitrakopoulou-Strauss A. PET-based molecular imaging in personalized oncology: potential of the assessment of therapeutic outcome. Future Oncol. 2015;11:1083–91.
Wahl RL. 2013 SNMMI highlights lecture: oncology. J Nucl Med. 2013;54:11N–22N.
Ribas A, Benz MR, Allen-Auerbach MS, Radu C, Chmielowski B, Seja E, et al. Imaging of CTLA4 blockade-induced cell replication with (18)F-FLT PET in patients with advanced melanoma treated with tremelimumab. J Nucl Med. 2010;51:340–6.
Breki C-M, Dimitrakopoulou-Strauss A, Hassel J, Theoharis T, Sachpekidis C, Pan L, et al. Fractal and multifractal analysis of PET/CT images of metastatic melanoma before and after treatment with ipilimumab. EJNMMI Res. 2016;6:61.
Kong BY, Menzies AM, Saunders CAB, Liniker E, Ramanujam S, Guminski A, et al. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res. 2016;29:572–7.
Cho SY, Lipson EJ, Im H-J, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point 18F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–8.
Wong AN, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):67–77.
Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with Ipilimumab. Cancer Immunol Res. 2015;3:1185–92.
Kähler KC, Hassel JC, Heinzerling L, Loquai C, Mössner R, Ugurel S, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14:662–81.
Wachsmann JW, Ganti R, Peng F. Immune-mediated disease in Ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol. 2017;24:111–5.
Funding
This study was supported in part by the German Cancer Aid under the project “Therapy monitoring of ipilimumab based on the quantification of F-18-FDG kinetics with 4D PET/CT (dPET-CT) in patients with melanoma (stage 4)”. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Julia Winkler received speakers honoraria from MSD, and travel support from AMGEN, BMS, MSD, Philochem and Roche. Jessica C. Hassel received honoraria for talks and travel expenses from BMS. The other authors declare no conflicts of interest.
Ethical approval
The study was approved by the Ethics Committee of the University of Heidelberg (S-107/2012) and the Federal Agency for Radiation Protection (Bundesamt für Strahlenschutz).
Informed consent
Informed consent was obtained from all individual participants included in the study. This study did not include any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Anwar, H., Sachpekidis, C., Winkler, J. et al. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 45, 376–383 (2018). https://doi.org/10.1007/s00259-017-3870-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3870-6