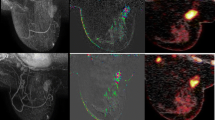
Abstract
Purpose
To explore whether integrated 18F-FDG PET/MRI can be used to predict pathological response to neoadjuvant chemotherapy (NAC) in patients with breast cancer.
Methods
Between November 2014 and April 2016, 26 patients with breast cancer who had received NAC and subsequent surgery were prospectively enrolled. Each patient underwent 18F-FDG PET/MRI examination before and after the first cycle of NAC. Qualitative MRI parameters, including morphological descriptors and the presence of peritumoral oedema were assessed. Quantitatively, PET parameters, including maximum standardized uptake value, metabolic tumour volume and total lesion glycolysis (TLG), and MRI parameters, including washout proportion and signal enhancement ratio (SER), were measured. The performance of the imaging parameters singly and in combination in predicting a pathological incomplete response (non-pCR) was assessed.
Results
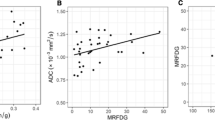
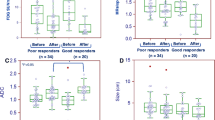
Of the 26 patients, 7 (26.9%) exhibited a pathological complete response (pCR), and 19 (73.1%) exhibited a non-pCR. No significant differences were found between the pCR and non-pCR groups in the qualitative MRI parameters. The mean percentage reductions in TLG30% on PET and SER on MRI were significantly greater in the pCR group than in the non-pCR group (TLG30% −64.8 ± 15.5% vs. −25.4 ± 48.7%, P = 0.005; SER −34.6 ± 19.7% vs. −8.7 ± 29.0%, P = 0.040). The area under the receiver operating characteristic curve for the percentage change in TLG30% (0.789, 95% CI 0.614 to 0.965) was similar to that for the percentage change in SER (0.789, 95% CI 0.552 to 1.000; P = 1.000).The specificity of TLG30% in predicting pCR) was 100% (7/7) and that of SER was 71.4% (5/7). The sensitivity of TLG30% in predicting non-pCR was 63.2% (12/19) and that of SER was 84.2% (16/19). When the combined TLG30% and SER criterion was applied, sensitivity was 100% (19/19), and specificity was 71.4% (5/7).
Conclusion
18F-FDG PET/MRI can be used to predict non-pCR after the first cycle of NAC in patients with breast cancer and has the potential to improve sensitivity by the addition of MRI parameters to the PET parameters.



Similar content being viewed by others
Abbreviations
- NAC:
-
neoadjuvant chemotherapy
- FDG PET:
-
18F-fluoro-deoxy-glucose positron emission tomography
- pCR:
-
pathological complete response
- non-pCR:
-
pathological incomplete response
- MRI:
-
magnetic resonance imaging
- SUV:
-
standardized uptake value
- MTV:
-
metabolic tumour volume
- TLG:
-
total lesion glycolysis
- LN:
-
lymph node
- SER:
-
signal enhancement ratio
- ER:
-
oestrogen receptor
- PR:
-
progesterone receptor
- HER:
-
human epidermal growth factor receptor
- RCB:
-
residual cancer burden
- ICC:
-
intraclass correlation coefficient
References
Graham PJ, Brar MS, Foster T, et al. Neoadjuvant chemotherapy for breast cancer, is practice changing? A population-based review of current surgical trends. Ann Surg Oncol. 2015;22:3376–82.
Rubovszky G, Horváth Z. Recent advances in the neoadjuvant treatment of breast cancer. J Breast Cancer. 2017;20:119–31.
von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
von Minckwitz G, Blohmer JU, Costa SD, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623–30.
Tabchy A, Valero V, Vidaurre T, et al. Evaluation of a 30-gene paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide chemotherapy response predictor in a multicenter randomized trial in breast cancer. Clin Cancer Res. 2010;16:5351–61.
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med. 2012;53:928–38.
Rousseau C, Devillers A, Sagan C, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–72.
Schwarz-Dose J, Untch M, Tiling R, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–41.
Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy – results from ACRIN 6657/I-SPY TRIAL. Radiology. 2012;263:663–72.
Ah-See ML, Makris A, Taylor NJ, et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008;14:6580–9.
Cho N, Im SA, Park IA, et al. Breast cancer: early prediction of response to neoadjuvant chemotherapy using parametric response maps for MR imaging. Radiology. 2014;272:385–96.
Avril S, Muzic RF Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. 18F-FDG PET/CT for monitoring of treatment response in breast cancer. J Nucl Med. 2016;57:34S–9S.
Cysouw MC, Kramer GM, Schoonmade LJ, et al. Impact of partial-volume correction in oncological PET studies: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2017. https://doi.org/10.1007/s00259-017-3775-4
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Lopci E, Zucali PA, Ceresoli GL, et al. Quantitative analyses at baseline and interim PET evaluation for response assessment and outcome definition in patients with malignant pleural mesothelioma. Eur J Nucl Med Mol Imaging. 2015;42:667–75.
Im HJ, Kim YK, Kim YI, Lee JJ, Lee WW, Kim SE. Usefulness of combined metabolic-volumetric indices of (18)F-FDG PET/CT for the early prediction of neoadjuvant chemotherapy outcomes in breast cancer. Nucl Med Mol Imaging. 2013;47:36–43.
Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston: American College of Radiology; 2013.
Esserman L, Kaplan E, Partridge S, et al. MRI phenotype is associated with response to doxorubicin and cyclophosphamide neoadjuvant chemotherapy in stage III breast cancer. Ann Surg Oncol. 2001;8:549–59.
Uematsu T. Focal breast edema associated with malignancy on T2-weighted images of breast MRI: peritumoral edema, prepectoral edema, and subcutaneous edema. Breast Cancer. 2015;22:66–70.
Bae MS, Shin SU, Ryu HS, et al. Pretreatment MR imaging features of triple-negative breast cancer: association with response to neoadjuvant chemotherapy and recurrence-free survival. Radiology. 2016;281:392–400.
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22.
Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049–60.
Tateishi U, Miyake M, Nagaoka T, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging – prospective assessment. Radiology. 2012;263:53–63.
Pengel KE, Koolen BB, Loo CE, et al. Combined use of 18F-FDG PET/CT and MRI for response monitoring of breast cancer during neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1515–24.
Lim I, Noh WC, Park J, et al. The combination of FDG PET and dynamic contrast-enhanced MRI improves the prediction of disease-free survival in patients with advanced breast cancer after the first cycle of neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1852–60.
Liu Q, Wang C, Li P, Liu J, Huang G, Song S. The role of (18)F-FDG PET/CT and MRI in assessing pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: a systematic review and meta-analysis. Biomed Res Int. 2016;2016:3746232.
Moy L, Noz ME, Maguire GQ Jr, et al. Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J. 2010;16:369–76.
Groheux D, Sanna A, Majdoub M, et al. Baseline tumor 18F-FDG uptake and modifications after 2 cycles of neoadjuvant chemotherapy are prognostic of outcome in ER+/HER2- breast cancer. J Nucl Med. 2015;56:824–31.
Specht JM, Kurland BF, Montgomery SK, et al. Tumor metabolism and blood flow as assessed by positron emission tomography varies by tumor subtype in locally advanced breast cancer. Clin Cancer Res. 2010;16:2803–10.
Mankoff DA, Dunnwald LK, Gralow JR, et al. Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med. 2002;43:500–9.
Dunnwald LK, Gralow JR, Ellis GK, et al. Tumor metabolism and blood flow changes by positron emission tomography: relation to survival in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol. 2008;26:4449–57.
Dunnwald LK, Doot RK, Specht JM, et al. PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res. 2011;17:2400–9.
Hatt M, Groheux D, Martineau A, et al. Comparison between 18F-FDG PET image-derived indices for early prediction of response to neoadjuvant chemotherapy in breast cancer. J Nucl Med. 2013;54:341–9.
Groheux D, Majdoub M, Sanna A, et al. Early metabolic response to neoadjuvant treatment: FDG PET/CT criteria according to breast cancer subtype. Radiology. 2015;277:358–71.
Li KL, Henry RG, Wilmes LJ, et al. Kinetic assessment of breast tumors using high spatial resolution signal enhancement ratio (SER) imaging. Magn Reson Med. 2007;58:572–81.
Funding
This study was funded by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2014R1A1A2053682), and by a grant (no. 30–2016-0110 and 30-2015-0130) from the Seoul National University Hospital. We also sincerely appreciate Mrs. Myung-Hwa Lee and Mr. Hyuk Jin Chung for their dedication to breast cancer research funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Cho, N., Im, SA., Cheon, G.J. et al. Integrated 18F-FDG PET/MRI in breast cancer: early prediction of response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 45, 328–339 (2018). https://doi.org/10.1007/s00259-017-3849-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3849-3