Abstract
Purpose
The molecular features isocitrate dehydrogenase (IDH) mutation and 1p/19q co-deletion have gained major importance for both glioma typing and prognosis and have, therefore, been integrated in the World Health Organization (WHO) classification in 2016. The aim of this study was to characterize static and dynamic O-(2-18F-fluoroethyl)-L-tyrosine (18F–FET) PET parameters in gliomas with or without IDH mutation or 1p/19q co-deletion.
Methods
Ninety patients with newly diagnosed and untreated gliomas with a static and dynamic 18F–FET PET scan prior to evaluation of tumor tissue according to the 2016 WHO classification were identified retrospectively. Mean and maximum tumor-to-brain ratios (TBRmean/max), as well as dynamic parameters (time-to-peak and slope) of 18F–FET uptake were calculated.
Results
Sixteen (18%) oligodendrogliomas (IDH mutated, 1p/19q co-deleted), 27 (30%) astrocytomas (IDH mutated only), and 47 (52%) glioblastomas (IDH wild type only) were identified. TBRmean, TBRmax, TTP and slope discriminated between IDH mutated astrocytomas and IDH wild type glioblastomas (P < 0.01). TBRmean showed the best diagnostic performance (cut-off 1.95; sensitivity, 89%; specificity, 67%; accuracy, 81%). None of the parameters discriminated between oligodendrogliomas (IDH mutated, 1p/19q co-deleted) and glioblastomas or astrocytomas. Furthermore, TBRmean, TBRmax, TTP, and slope discriminated between gliomas with and without IDH mutation (p < 0.01). The best diagnostic performance was obtained for the combination of TTP with TBRmax or slope (accuracy, 73%).
Conclusion
Data suggest that static and dynamic 18F–FET PET parameters may allow determining non-invasively the IDH mutation status. However, IDH mutated and 1p/19q co-deleted oligodendrogliomas cannot be differentiated from glioblastomas and astrocytomas by 18F–FET PET.
Similar content being viewed by others
Introduction
In addition to standard magnetic resonance imaging (MRI), positron emission tomography (PET) using radiolabeled amino acids has recently been recommended by the Response Assessment in Neuro-Oncology (RANO) working group for glioma diagnostics [1]. Among amino acid PET tracers, O-(2-18F-fluoroethyl)-L-tyrosine (18F–FET) is a tracer of great interest for non-invasive grading [2], prognostication [3], differential diagnosis [4], delineation of tumor extent [5, 6], planning of stereotactic biopsy, glioma resection and radiotherapy [7, 8], and treatment monitoring [9,10,11].
In terms of patient stratification with an improved prognosis, the ability of dynamic 18F–FET PET for prognostication has been described for low-grade [12], as well as for high-grade gliomas [3]. In these studies, typing of gliomas was performed according to the World Health Organization (WHO) 2007 classification [13]. However, more recent analyses of larger glioma cohorts have identified a dominant association of molecular features with clinical outcome [14]. Consequently, the WHO classification for gliomas has been updated in 2016 and molecular features for subclassification have been integrated [15]. Glioma patients with a gene mutation encoding for the isocitrate dehydrogenase enzyme (IDH) have a different biology and better outcome than patients with IDH wild type gliomas. IDH mutated gliomas are further subdivided into 1p/19q co-deleted (oligodendrogliomas, associated with an improved outcome) and 1p/19q intact gliomas (astrocytomas with an intermediate outcome). In contrast, IDH wild type glioblastomas have a poor outcome.
Up to now, only three studies have evaluated amino acid PET uptake characteristics in gliomas, in which both the IDH mutation status and presence of a 1p/19q co-deletion were obtained. The first study, performed with 11C–methyl-L-methionine (11C–MET) PET in 109 patients with newly diagnosed gliomas, showed an inverse correlation between 11C–MET uptake and presence of IDH mutation [16], indicating that IDH wild type gliomas (glioblastomas) had a significantly higher tracer uptake than IDH mutated gliomas. In contrast, a subsequent study involving 43 patients with newly diagnosed lower-grade gliomas (WHO grades II and III) examined using 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (18F–FDOPA) PET paradoxically revealed a higher 18F–FDOPA uptake in gliomas with IDH mutation [17], suggesting potential discrepancies between uptake patterns of different amino acid PET tracers in gliomas [18]. Another study evaluated 65 patients with low-grade gliomas only (WHO grades I and II) using static 18F–FET PET. No correlation between 18F–FET uptake and IDH mutation status could be observed, but the lack of 18F–FET uptake excluded a 1p/19q co-deletion with a high negative predictive value [19].
In this context, the aim of this study was to evaluate characteristics of static and dynamic 18F–FET PET parameters in a large cohort of newly diagnosed gliomas in which—according to the 2016 WHO classification—the IDH mutation status and the presence of a 1p/19 co-deletion was obtained.
Patients and methods
Patients
From October 2012 to September 2016, patients with newly diagnosed supratentorial glioma, who were referred to our centre for 18F–FET PET imaging prior to neuropathological confirmation of diagnosis and for whom a neuropathological report on the molecular features IDH mutation and 1p/19q co-deletion status was available, were retrospectively identified. The examined patients were consecutively sent for differential diagnosis or in order to identify the metabolically most active tumor parts for biopsy planning. The time window between PET imaging and neuropathological confirmation was limited to 100 days for lower-grade astrocytomas and oligodendrogliomas, and to 30 days for glioblastomas.
The ethics committee of the University of Aachen approved the retrospective data evaluation. There was no conflict with the Declaration of Helsinki. All subjects had given prior written informed consent for the PET and MRI investigations and the use of the data for scientific evaluations.
PET imaging
The amino acid 18F–FET was produced and applied as described previously [7]. According to the German guidelines for brain tumor imaging using labeled amino acid analogues; all patients fasted for at least 12 h before PET acquisition [20]. Dynamic PET scans were acquired up to 40 min after intravenous injection of 2.8 ± 0.3 MBq of 18F–FET/kg of body weight. PET imaging was performed either on an ECAT Exact HR+ PET scanner in 3-dimensional mode (Siemens Medical Systems) (axial field of view, 15.5 cm; spatial resolution, 6 mm) or simultaneously with MR imaging using a BrainPET insert. The BrainPET is a compact cylinder that fits in the bore of the Magnetom Trio MR scanner (axial field of view, 19.2 cm; optimum spatial resolution, 3 mm) [21]. Forty-eight patients had their examination on the ECAT HR+ PET scanner, whereas 42 patients were scanned using the PET/MR system (no significant differences within either group, s. Tab. 1). Iterative reconstruction parameters were 16 subsets, six iterations using the OSEM algorithm for ECAT HR+ PET scanner and two subsets, 32 iterations using the OP-OSEM algorithm provided by the manufacturer for the BrainPET, with correction for random, scattered coincidences, and dead time for both systems. Attenuation correction for the ECAT HR+ PET scan was based on a transmission scan, and for the BrainPET scan on a template-based approach [21]. For the evaluation of 18F–FET uptake, summed PET images over the period of 20–40 min post-injection were used for static data. The reconstructed dynamic data set consisted of 14 time frames (5 × 1 min; 5 × 3 min; 4 × 5 min).
Post-processing of PET images
Mean tumoural 18F–FET uptake was determined by a two-dimensional auto-contouring process using a tumor-to-brain ratio (TBR) of at least 1.6. This cut-off was based on a biopsy-controlled study in cerebral gliomas and differentiated best between tumoural and peritumoural tissue [6]. In order to exclude an influence of different scanner resolution of the HR+ scanner and the BrainPET scanner, a circular region of interest (ROI) with a diameter of 1.6 cm was centered on maximal tumor uptake [22] for the evaluation of the maximal 18F–FET uptake. If 18F–FET uptake in the lesion was similar to that in the healthy-appearing brain tissue, an ROI was placed manually on the area of signal abnormality in the T1- and T2-weighted transversal MR scan and transferred to the co-registered 18F–FET PET scan. Mean and maximum TBR (TBRmean and TBRmax) were calculated by dividing the mean and maximum standardized uptake value (SUV) of the tumor ROI by the mean SUV of a larger crescent shape ROI placed in the semioval centre of the contralateral unaffected hemisphere including white and gray matter [21].
Furthermore, time-activity curves (TAC) of 18F–FET uptake in the tumor were obtained using a circular ROI with a diameter of 16 mm centered on the maximal 18F–FET uptake that was applied to the entire dynamic dataset. Derived from TAC, time-to-peak values (TTP; time in minutes from the beginning of the dynamic acquisition up to the maximum SUV of the lesion) were calculated. Additionally, the slope of the TAC in the late phase of 18F–FET uptake was quantified by fitting a linear regression line to the late phase of the curve (20–50 min post-injection). As described previously, the slope was expressed in change of SUV per hour [23].
Neuropathological evaluation
After obtaining tumor tissue by surgery or stereotactic biopsy, all lesions were histologically classified according to the WHO classification 2016 of tumors of the central nervous system. For IDH mutation status, presence of an IDH1R132H protein expression was evaluated by immunohistochemistry. If immunostaining was negative, IDH was directly sequenced. The 1p/19q co-deletion status was analyzed by fluorescence in situ hybridization.
Statistical analysis
Descriptive statistics are provided as mean and standard deviation and/or median and range and categorical variables as percentage. For comparison of mean values of quantitative variables between two groups, Student t-tests were performed, with adjustment in case of unequal variances determined by Levene’s Test. ANOVA tests were performed to compare mean values of quantitative variables with normal distribution between three groups and Kruskal-Wallis tests in case of unequal variance distribution. Chi-squared tests were applied for comparing intergroup distribution of categorical variables. The diagnostic performance of the 18F–FET PET parameters TBRmax and TBRmean, TTP, and slope for the differentiation between IDH and 1p/19q status was assessed by receiver operating characteristic (ROC) curve analyses using the neuropathological results as reference. Decision cut-off was considered optimal when the product of paired values for sensitivity and specificity reached its maximum. A p-value below 0.05 was considered significant. Statistical analyses were performed using SPSS Statistics 20 software (SPSS Inc., Chicago, IL, USA).
Results
Patients
In total, 27 (30%) IDH mutated astrocytomas (8 diffuse astrocytomas, WHO grade II; 19 anaplastic astrocytomas, WHO grade III), 16 (18%) IDH mutated and 1p/19q co-deleted oligodendrogliomas (eight diffuse oligodendrogliomas, WHO grade II; eight anaplastic oligodendrogliomas, WHO grade III), and 47 (52%) IDH wild type glioblastomas were identified retrospectively. Thus, 43 patients were IDH mutated (48%), while 47 patients (52%) had an IDH wild type. The mean age of these 90 patients was 51.3 ± 13.7 years (35 women). Patient characteristics of the three groups with different molecular features are listed in Table 1.
Static 18F–FET PET data
Both TBRmean and TBRmax discriminated between IDH mutated astrocytomas and IDH wild type glioblastomas (cut-off for TBRmean, 1.95; sensitivity, 89%; specificity, 67%; and accuracy, 81%; p < 0.01) (cut-off for TBRmax, 1.95; sensitivity, 91%; specificity, 59%; and accuracy; 80%; p < 0.01) (Figs. 1 and 2).
Dynamic 18F–FET PET data and combined analysis
Both TTP and slope discriminated between IDH mutated astrocytomas and IDH wild type glioblastomas (TTP cut-off, 30 min; sensitivity, 72%; specificity; 81%; accuracy, 76%; p < 0.01) (slope cut-off, −0.02 SUV/h; sensitivity, 74%; specificity, 70%; accuracy, 73%; p < 0.01) (Figs. 3 and 4). Combined analysis did not improve the diagnostic performance of TBRmean for the discrimination between IDH mutated astrocytomas and IDH wild type glioblastomas.
Furthermore, none of the parameters discriminated between oligodendrogliomas (IDH mutated, 1p/19q co-deleted) and glioblastomas or astrocytomas.
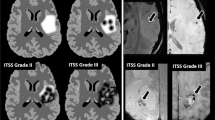
Imaging examples of gliomas from each group are provided in Fig. 5.
Axial slices of contrast-enhanced T1-weighted MRI (left row), 18F–FET PET (middle row) and time-activity curves of 18F–FET uptake with a dotted red line for the definition of time-to-peak and a dotted orange line for the linear regression of the slope (right row). The upper panel shows a 33-year-old woman with an IDH mutated anaplastic astrocytoma (WHO grade III; TBRmean of 1.7, TBRmax of 2.1, time-to-peak of 37.5 min, and slope of 0.26 SUV/h). The middle panel shows a 60-year-old man with a diffuse oligodendroglioma (WHO grade II, IDH mutated, 1p/19q co-deleted; TBRmean of 2.2, TBRmax of 5.2, time-to-peak of 37.5 min, and slope of 1.12 SUV/h). The lower panel shows a 32-year-old woman with an IDH wild-type glioblastoma (TBRmean of 3.4, TBRmax of 8.8, time-to-peak of 6.5 min, and a slope of −0.83 SUV/h)
IDH mutation status
TBRmean, TBRmax, TTP, and slope showed significant differences between gliomas with and without IDH mutation (p < 0.01). The best diagnostic performance was obtained with the combination of TTP with TBRmax or slope, achieving accuracies with both parameters of 73%. Diagnostic performances for each parameter and combined are presented in Table 2.
Discussion
In view of the 2016 WHO classification, this study shows that static and dynamic 18F–FET PET parameters are helpful to discriminate between IDH mutated astrocytomas and IDH wild type glioblastomas, two of the most common groups of gliomas with different prognosis. Moreover, data suggest that static and dynamic 18F–FET PET imaging may allow non-invasive determination of the IDH mutation status. However, data also show that another important group of gliomas—IDH mutated and 1p/19q co-deleted oligodendrogliomas—remains indistinguishable from glioblastomas and astrocytomas when applying static and dynamic 18F–FET PET.
Recently, 18F–FET PET has evoked great interest for non-invasive glioma grading according to the 2007 WHO classification [4, 12, 13]. In a series of 77 low-grade gliomas and 66 high-grade gliomas, static 18F–FET PET parameters discriminated between both groups with an accuracy of 72% [4]. Furthermore, in case of newly diagnosed MRI-suspected low-grade gliomas, a high sensitivity of detection for anaplastic foci was observed with dynamic 18F–FET PET [12]. Accordingly, the diagnostic performance for grading with 18F–FET PET could be improved using dynamic data [24,25,26]. For example, TAC in low-grade gliomas usually show a steadily increasing 18F–FET uptake with a late TTP, whereas high-grade gliomas frequently show an early TTP within the first 10–20 min followed by a descent of the TAC [24, 26]. These dynamic analyses led to an improved diagnostic accuracy for the differentiation of low-grade from high-grade gliomas with accuracies of about 80 to 90% [24, 26]. Furthermore, recent studies suggest that dynamic 18F–FET PET is also helpful for the non-invasive assessment of the individual prognosis in newly diagnosed low-grade and high-grade gliomas [3, 27,28,29] and for the detection of malignant progression in low-grade gliomas [28]. Nevertheless, it should be noted that uptake intensities in gliomas may vary considerably resulting in a large overlap between different WHO grades according to the 2007 classification [24]. Moreover, based on histology according to the 2007 WHO classification, an oligodendroglioma will be diagnosed independent of 1p/19q co-deletion. In contrast, based on the 2016 WHO classification, oligodendrogliomas without a 1p/19q co-deletion are classified as astrocytomas. This is of great clinical relevance because the prognosis of oligodendrogliomas and astrocytomas may vary considerably. Furthermore, particularly low-grade oligodendrogliomas may show a clearly higher amino acid uptake [30, 31]. Thus, it is not surprising that diagnostic performances of TBRmax, TBRmean and TTP for the discrimination between IDH mutated astrocytomas and IDH wild type glioblastomas in the present study are better than those of our previous published studies based on the WHO 2007 classification for the discrimination between low-grade and high-grade gliomas (respective accuracies of 80, 81, and 76% vs. 71, 72 and 69%) [4, 26]. Based on the improved diagnosis of oligodendrogliomas it seems that the 2016 WHO classification more accurately reflects the metabolic tumor activity measured by FET PET.
Up to now, amino acid PET characteristics in gliomas classified by the 2016 WHO classification are not well described in the literature. This revised classification now integrates molecular features, particularly giving pivotal attention to the IDH mutation and 1p/19q co-deletion, which are crucial factors for an improved prognosis [15] and a predictive marker for the individual’s response to chemoradiation [32, 33]. Furthermore, the IDH mutation status allows an improved assessment of prognosis than the WHO grades defined by the 2007 classification [34, 35].
Our current results resemble those observed in a 11C–MET PET study showing an inverse correlation between tracer uptake and the presence of an IDH mutation [16]. Taken together, the latter study and the present study suggest that IDH wild type gliomas such as glioblastomas have a significantly higher tracer uptake than IDH mutated gliomas [16]. This is, however, in contrast to a previous amino acid PET study using 18F–FDOPA, in which a higher amino acid uptake was observed in IDH mutated gliomas only [17], suggesting potential discrepancies within different amino acid PET tracers [18]. It should be noted that this finding was observed in lower-grade gliomas only (WHO grades II and III), with a high proportion of oligodendrogliomas in IDH mutated tumors (35%). However, glioblastomas were not evaluated. Another study has also evaluated relationships with IDH mutation status in low-grade gliomas (WHO grades I and II only) [19]. Again, no correlation between 18F–FET uptake and IDH mutation status was observed, but a lack of 18F–FET uptake excluded a 1p/19q co-deletion with a high negative predictive value. Unfortunately, however, in that study dynamic 18F–FET PET data were not available [19].
Extending the latter studies, the present data suggest that static and dynamic 18F–FET PET parameters discriminate between two of the most common groups of gliomas according the 2016 WHO classification 36, i.e., IDH mutated astrocytomas and IDH wild type glioblastomas. Interestingly, the group of oligodendrogliomas with IDH-mutant and 1p/19q co-deletion showed an overlap with other groups in TBRs, TTP, and also slope, which is in accordance with difficulties to individualize oligodendroglial component in gliomas, as reported in the studies mentioned above [30, 31].
Another finding of the present study is that 18F–FET PET may be helpful for the non-invasive identification of the IDH mutation status. Non-invasive imaging parameters in gliomas have been recently developed and might support clinical decision-making. For example, the integration of 2-hydroxyglutarate proton magnetic resonance spectroscopy (2-HG MRS) into clinical practice for treatment monitoring in IDH-mutant gliomas has been evaluated: sensitivity of detection was, however, highly variable and dependent on tumor volume, ranging from 8 to 91% [36]. Furthermore, 2-HG MRS is not easily implemented into clinical routine. In the current study, the combination of static and dynamic 18F–FET PET parameters was helpful to differentiate IDH-mutated from IDH-wild type gliomas, albeit that the diagnostic performance was moderate with an accuracy of 73%. Results warrant further exploration whether diagnostic accuracy can be enhanced.
The following limitations need to be considered. First, groups of IDH wild type astrocytomas and IDH mutated glioblastomas were not included in the study. However, in the general population the incidence of gliomas with these molecular features is very rare [37], and there were no or too few datasets available for a meaningful analysis and/or inclusion in the present study. Second, for PET imaging two scanners with different spatial resolution were used. Despite our methodical efforts to exclude an influence of different scanner resolution, the optimal approach would be to have all cases studied on the same scanner. Third, in addition to the retrospective nature of the present study, no follow-up data were available for the present study, thereby not allowing the calculation of overall survival for each group separately. However, the main aim of this study was to evaluate baseline characteristics of static and dynamic 18F–FET PET parameters in a large cohort of newly diagnosed gliomas classified according to the 2016 WHO classification. This limitation may be addressed in a future study.
In summary, using static and dynamic 18F–FET PET parameters this study aimed to characterize newly diagnosed gliomas, which were classified according to the 2016 WHO classification. Static and dynamic 18F–FET PET data showed significant diagnostic value to discriminate between IDH mutated astrocytomas and IDH wild type glioblastomas, two of the most common groups of gliomas. In contrast, IDH mutated and 1p/19q co-deleted oligodendrogliomas showed a high overlap in static and dynamic 18F–FET PET data with the other two groups. Data suggest that static and dynamic 18F–FET PET may enable the non-invasive determination of the IDH mutation status. Prospective studies are now warranted to investigate this further.
References
Albert NL, Weller M, Suchorska B, et al. Response assessment in Neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology. 2016;18(9):1199–208.
Albert NL, Winkelmann I, Suchorska B, et al. Early static (18)F-FET-PET scans have a higher accuracy for glioma grading than the standard 20-40 min scans. Eur J Nucl Med Mol Imaging. 2016;43(6):1105–14.
Jansen NL, Suchorska B, Wenter V, et al. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med. 2015;56(1):9–15.
Rapp M, Heinzel A, Galldiks N, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54(2):229–35.
Piroth MD, Holy R, Pinkawa M, et al. Prognostic impact of postoperative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol. 2011;99(2):218–24.
Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–87.
Galldiks N, Langen K-J, Holy R, et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med. 2012 Jul;53(7):1048–57.
Suchorska B, Jansen NL, Linn J, et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84(7):710–9.
Wyss M, Hofer S, Bruehlmeier M, et al. Early metabolic responses in temozolomide treated low-grade glioma patients. J Neuro-Oncol. 2009;95(1):87–93.
Galldiks N, Rapp M, Stoffels G, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33.
Galldiks N, Law I, Pope WB, Arbizu J, Langen K-J. The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. NeuroImage Clin. 2017;13:386–94.
Jansen NL, Graute V, Armbruster L, et al. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging. 2012;39(6):1021–9.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl). 2007;114(2):97–109.
Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–508.
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (Berl). 2016;131(6):803–20.
Lopci E, Riva M, Olivari L, et al. Prognostic value of molecular and imaging biomarkers in patients with supratentorial glioma. Eur J Nucl Med Mol Imaging. 2017;44(7):1155–64.
Verger A, Metellus P, Sala Q, et al. IDH mutation is paradoxically associated with higher 18F-FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur J Nucl Med Mol Imaging. 2017;44(8):1306–11.
Verger A, Taieb D, Guedj E. Is the information provided by amino acid PET radiopharmaceuticals clinically equivalent in gliomas? Eur J Nucl Med Mol Imaging. 2017;44(8):1408–10.
Bette S, Gempt J, Delbridge C, et al. Prognostic value of O-(2-[18F]-fluoroethyl)-L-tyrosine-positron emission tomography imaging for histopathologic characteristics and progression-free survival in patients with low-grade glioma. World Neurosurg. 2016;89:230–9.
Langen K-J, Bartenstein P, Boecker H, et al. German guidelines for brain tumour imaging by PET and SPECT using labelled amino acids. Nukl Nucl Med. 2011;50(4):167–73.
Herzog H, Langen K-J, Weirich C, et al. High resolution BrainPET combined with simultaneous MRI. Nukl Nucl Med. 2011;50(2):74–82.
Galldiks N, Stoffels G, Filss C, et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro-Oncology. 2015;17(9):1293–300.
Ceccon G, Lohmann P, Stoffels G, et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro-Oncology. 2017;19(2):281–8.
Pöpperl G, Kreth FW, Mehrkens JH, et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34(12):1933–42.
Kunz M, Thon N, Eigenbrod S, et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro-Oncology. 2011;13(3):307–16.
Lohmann P, Herzog H, Rota Kops E, et al. Dual-time-point O-(2-[(18)F]fluoroethyl)-L-tyrosine PET for grading of cerebral gliomas. Eur Radiol. 2015;25(10):3017–24.
Floeth FW, Pauleit D, Sabel M, et al. Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med. 2007;48(4):519–27.
Galldiks N, Stoffels G, Ruge MI, et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med. 2013;54(12):2046–54.
Thon N, Kunz M, Lemke L, et al. Dynamic 18 F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses: dynamic PET in suspected low-grade gliomas. Int J Cancer. 2015;136(9):2132–45.
Jansen NL, Schwartz C, Graute V, et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [(18)F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro-Oncology. 2012;14(12):1473–80.
Manabe O, Hattori N, Yamaguchi S, et al. Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur J Nucl Med Mol Imaging. 2015;42(6):896–904.
van den Bent MJ, Brandes AA, Taphoorn MJB, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–50.
Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783–90.
Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol (Berl). 2015;129(6):867–73.
Olar A, Wani KM, Alfaro-Munoz KD, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol (Berl). 2015;129(4):585–96.
de la Fuente MI, Young RJ, Rubel J, et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro-Oncology. 2016;18(2):283–90.
Jiang H, Cui Y, Wang J, Lin S. Impact of epidemiological characteristics of supratentorial gliomas in adults brought about by the 2016 world health organization classification of tumors of the central nervous system. Oncotarget. 2017;8(12):20354–61.
Funding
This work was supported by the Wilhelm-Sander Stiftung, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Verger, A., Stoffels, G., Bauer, E.K. et al. Static and dynamic 18F–FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur J Nucl Med Mol Imaging 45, 443–451 (2018). https://doi.org/10.1007/s00259-017-3846-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3846-6