Abstract
Purpose
To investigate the utility of Pittsburgh compound B (PiB) positron emission tomography (PET) imaging for evaluating whole-body amyloid involvement in patients with systemic amyloidosis.
Methods
Whole-body 11C-PiB PET was performed in seven patients with systemic immunoglobulin light-chain (AL) amyloidosis, seven patients with hereditary transthyretin (ATTRm) amyloidosis, one asymptomatic TTR mutation carrier and three healthy controls. The correlations between clinical organ involvement, radiological 11C-PiB uptake and histopathological findings were analysed for each organ.
Results
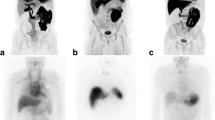
Organ involvement on 11C-PiB PET imaging showed good correlations with the clinical findings for the heart and stomach. Abnormal tracer uptake was also observed in the spleen, lachrymal gland, submandibular gland, sublingual gland, lymph node, brain, scalp, extraocular muscles, nasal mucosa, pharynx, tongue and nuchal muscles, most of which were asymptomatic. Physiological tracer uptake was universally observed in the urinary tract (kidney, renal pelvis, ureter and bladder) and enterohepatic circulatory system (liver, gallbladder, bile duct and small intestine) in all participants. Most of the patients and one healthy control subject showed asymptomatic tracer uptake in the lung and parotid gland. The peripheral nervous system did not show any tracer uptake even in patients with apparent peripheral neuropathy. Histological amyloid deposition was confirmed in biopsied myocardium and gastric mucosa where abnormal 11C-PiB retention was observed.
Conclusions
11C-PiB PET imaging can be used clinically in the systemic evaluation of amyloid distribution in patients with AL and ATTRm amyloidosis. Quantitative analysis of 11C-PiB PET images may be useful in therapy evaluation and will reveal whether amyloid clearance is correlated with clinical response.



Similar content being viewed by others
References
Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2013;40:104–14. https://doi.org/10.1007/s00259-012-2237-2.
Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–34. https://doi.org/10.1002/ana.21164.
LeVine H III. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–84.
Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjo L, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med. 2013;54:213–20. https://doi.org/10.2967/jnumed.111.102053.
Hellstrom-Lindahl E, Westermark P, Antoni G, Estrada S. In vitro binding of [3H]PIB to human amyloid deposits of different types. Amyloid. 2014;21:21–7. https://doi.org/10.3109/13506129.2013.860895.
Sekijima Y, Yazaki M, Oguchi K, Ezawa N, Yoshinaga T, Yamada M, et al. Cerebral amyloid angiopathy in posttransplant patients with hereditary ATTR amyloidosis. Neurology. 2016;87:773–81. https://doi.org/10.1212/wnl.0000000000003001.
Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004, 18-22 April 2004. Am J Hematol. 2005;79:319–28. https://doi.org/10.1002/ajh.20381.
Sekijima Y, Uchiyama S, Tojo K, Sano K, Shimizu Y, Imaeda T, et al. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011;42:1785–91. https://doi.org/10.1016/j.humpath.2011.03.004.
Scheinin NM, Tolvanen TK, Wilson IA, Arponen EM, Nagren KA, Rinne JO. Biodistribution and radiation dosimetry of the amyloid imaging agent 11C-PIB in humans. J Nucl Med. 2007;48:128–33.
Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323:508–13. https://doi.org/10.1056/nejm199008233230803.
Rydh A, Suhr O, Hietala SO, Ahlstrom KR, Pepys MB, Hawkins PN. Serum amyloid P component scintigraphy in familial amyloid polyneuropathy: regression of visceral amyloid following liver transplantation. Eur J Nucl Med. 1998;25:709–13.
Nelson SR, Hawkins PN, Richardson S, Lavender JP, Sethi D, Gower PE, et al. Imaging of haemodialysis-associated amyloidosis with 123I-serum amyloid P component. Lancet. 1991;338:335–9.
Rowczenio D, Dogan A, Theis JD, Vrana JA, Lachmann HJ, Wechalekar AD, et al. Amyloidogenicity and clinical phenotype associated with five novel mutations in apolipoprotein A-I. Am J Pathol. 2011;179:1978–87. https://doi.org/10.1016/j.ajpath.2011.06.024.
Hutt DF, Gilbertson J, Quigley AM, Wechalekar AD. (99m)Tc-DPD scintigraphy as a novel imaging modality for identification of skeletal muscle amyloid deposition in light-chain amyloidosis. Amyloid. 2016;23:134–5. https://doi.org/10.3109/13506129.2016.1158160.
Puille M, Altland K, Linke RP, Steen-Muller MK, Kiett R, Steiner D, et al. 99mTc-DPD scintigraphy in transthyretin-related familial amyloidotic polyneuropathy. Eur J Nucl Med Mol Imaging. 2002;29:376–9.
Quarta CC, Obici L, Guidalotti PL, Pieroni M, Longhi S, Perlini S, et al. High 99mTc-DPD myocardial uptake in a patient with apolipoprotein AI-related amyloidotic cardiomyopathy. Amyloid. 2013;20:48–51. https://doi.org/10.3109/13506129.2012.746938.
Rao BK, Padmalatha C, Au Buchon J, Lieberman LM. Hepatic and splenic scintigraphy in idiopathic systemic amyloidosis. Eur J Nucl Med. 1981;6:143–6.
Janssen S, Piers DA, van Rijswijk MH, Meijer S, Mandema E. Soft-tissue uptake of 99mTc-diphosphonate and 99mTc-pyrophosphate in amyloidosis. Eur J Nucl Med. 1990;16:663–70.
Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. https://doi.org/10.1161/circimaging.112.000132.
Nakagawa M, Sekijima Y, Yazaki M, Tojo K, Yoshinaga T, Doden T, et al. Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid. 2016;23:58–63. https://doi.org/10.3109/13506129.2015.1135792.
Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, et al. Imaging cardiac amyloidosis: a pilot study using 18F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41:1652–62. https://doi.org/10.1007/s00259-014-2787-6.
Osborne DR, Acuff SN, Stuckey A, Wall JS. A routine PET/CT protocol with streamlined calculations for assessing cardiac amyloidosis using (18)F-florbetapir. Front Cardiovasc Med. 2015;2:23. https://doi.org/10.3389/fcvm.2015.00023.
Broski SM, Spinner RJ, Howe BM, Dispenzieri A, Johnson GB. 18F-Florbetapir and 18F-FDG PET/CT in systemic immunoglobulin light chain amyloidosis involving the peripheral nerves. Clin Nucl Med. 2016;41:e115–7. https://doi.org/10.1097/rlu.0000000000000947.
García-González P, Sánchez-Jurado R, Cozar-Santiago MP, Ferrando-Beltrán M, Pérez-Rodriguez PL, Ferrer-Rebolleda J. Laryngeal and cardiac amyloidosis diagnosed by 18F-Florbetapir PET/CT. Rev Esp Med Nucl Imagen Mol. 2017;36:135–6. https://doi.org/10.1016/j.remn.2016.03.006.
Leung N, Ramirez-Alvarado M, Nasr SH, Kemp BJ, Johnson GB. Detection of ALECT2 amyloidosis by positron emission tomography-computed tomography imaging with florbetapir. Br J Haematol. 2017;177:12. https://doi.org/10.1111/bjh.14519.
D'Estanque E, Chambert B, Moranne O, Kotzki PO, Boudousq V. 18F-Florbetaben: a new tool for amyloidosis staging? Clin Nucl Med. 2017;42:50–3. https://doi.org/10.1097/rlu.0000000000001434.
Aprile C, Marinone G, Saponaro R, Bonino C, Merlini G. Cardiac and pleuropulmonary AL amyloid imaging with technetium-99m labelled aprotinin. Eur J Nucl Med. 1995;22:1393–401.
Schaadt BK, Hendel HW, Gimsing P, Jonsson V, Pedersen H, Hesse B. 99mTc-aprotinin scintigraphy in amyloidosis. J Nucl Med. 2003;44:177–83.
Hawkins PN, Pepys MB. Imaging amyloidosis with radiolabelled SAP. Eur J Nucl Med. 1995;22:595–9.
Bokhari S, Shahzad R, Castano A, Maurer MS. Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol. 2014;21:175–84. https://doi.org/10.1007/s12350-013-9803-2.
Ohyama T, Shimokama T, Yoshikawa Y, Watanabe T. Splenic amyloidosis: correlations between chemical types of amyloid protein and morphological features. Mod Pathol. 1990;3:419–22.
Martins AC, Rosa AM, Costa E, Tavares C, Quadrado MJ, Murta JN. Ocular manifestations and therapeutic options in patients with familial amyloid polyneuropathy: a systematic review. Biomed Res Int. 2015;2015:282405. https://doi.org/10.1155/2015/282405.
Hachulla E, Janin A, Flipo RM, Saile R, Facon T, Bataille D, et al. Labial salivary gland biopsy is a reliable test for the diagnosis of primary and secondary amyloidosis. A prospective clinical and immunohistologic study in 59 patients. Arthritis Rheum. 1993;36:691–7.
de Paula EF, de Mello BL, de Carvalho DL, Della-Guardia B, de Almeida MD, Marins LV, et al. Minor salivary gland biopsy for the diagnosis of familial amyloid polyneuropathy. Neurol Sci. 2017;38:311–8. https://doi.org/10.1007/s10072-016-2760-1.
Villa F, Dionigi G, Tanda ML, Rovera F, Boni L. Amyloid goiter. Int J Surg. 2008;6(Suppl 1):S16–8. https://doi.org/10.1016/j.ijsu.2008.12.025.
Matsuda M, Gono T, Shimojima Y, Yoshida T, Katoh N, Hoshii Y, et al. AL amyloidosis manifesting as systemic lymphadenopathy. Amyloid. 2008;15:117–24. https://doi.org/10.1080/13506120802006047.
Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59.
Matsuda M, Katoh N, Ikeda S. Clinical manifestations at diagnosis in Japanese patients with systemic AL amyloidosis: a retrospective study of 202 cases with a special attention to uncommon symptoms. Intern Med. 2014;53:403–12.
Funding
This study was funded by a Grant-in-aid for Scientific Research (C) (23,591,237 to Y.S.) from the Japan Society for the Promotion of Science, a grant from the Amyloidosis Research Committee, the Ministry of Health, Labour and Welfare, Japan, and 2015 Global ASPIRE TTR-FAP Competitive Research Grant Award from Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ezawa, N., Katoh, N., Oguchi, K. et al. Visualization of multiple organ amyloid involvement in systemic amyloidosis using 11C-PiB PET imaging. Eur J Nucl Med Mol Imaging 45, 452–461 (2018). https://doi.org/10.1007/s00259-017-3814-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3814-1