Abstract
Purpose
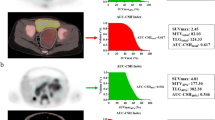
Risk-adapted treatment in children with neuroblastoma (NB) is based on clinical and genetic factors. This study evaluated the metabolic tumour volume (MTV) and its asphericity (ASP) in pretherapeutic 123I-MIBG SPECT for individualized image-based prediction of outcome.
Methods
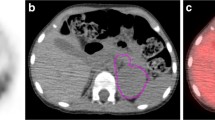
This retrospective study included 23 children (11 girls, 12 boys; median age 1.8 years, range 0.3–6.8 years) with newly diagnosed NB consecutively examined with pretherapeutic 123I-MIBG SPECT. Primary tumour MTV and ASP were defined using semiautomatic thresholds. Cox regression analysis, receiver operating characteristic analysis (cut-off determination) and Kaplan-Meier analysis with the log-rank test for event-free survival (EFS) were performed for ASP, MTV, laboratory parameters (including urinary homovanillic acid-to-creatinine ratio, HVA/C), and clinical (age, stage) and genetic factors. Predictive accuracy of the optimal multifactorial model was determined in terms of Harrell’s C and likelihood ratio χ 2.
Results
Median follow-up was 36 months (range 7–107 months; eight patients showed disease progression/relapse, four patients died). The only significant predictors of EFS in the univariate Cox regression analysis were ASP (p = 0.029; hazard ratio, HR, 1.032 for a one unit increase), MTV (p = 0.038; HR 1.012) and MYCN amplification status (p = 0.047; HR 4.67). The mean EFS in patients with high ASP (>32.0%) and low ASP were 21 and 88 months, respectively (p = 0.013), and in those with high MTV (>46.7 ml) and low MTV were 22 and 87 months, respectively (p = 0.023). A combined risk model of either high ASP and high HVA/C or high MTV and high HVA/C best predicted EFS.
Conclusions
In this exploratory study, pretherapeutic image-derived and laboratory markers of tumoral metabolic activity in NB (ASP, MTV, urinary HVA/C) allowed the identification of children with a high and low risk of progression/relapse under current therapy.




Similar content being viewed by others
References
Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–85.
Mueller S, Matthay KK. Neuroblastoma: biology and staging. Curr Oncol Rep. 2009;11:431–8.
Society for Paediatric Oncology and Haematology. NB2004 Trial Protocol for Risk Adapted Treatment of Children with Neuroblastoma. Version 1.00. Berlin: Society for Paediatric Oncology and Haematology; 2004.
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol. 2009;27:1007–13.
Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11.
Trahair TN, Vowels MR, Johnston K, Cohn RJ, Russell SJ, Neville KA, et al. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone Marrow Transplant. 2007;40:741–6.
Applebaum MA, Henderson TO, Lee SM, Pinto N, Volchenboum SL, Cohn SL. Second malignancies in patients with neuroblastoma: the effects of risk-based therapy. Pediatr Blood Cancer. 2015;62:128–33.
Decarolis B, Schneider C, Hero B, Simon T, Volland R, Roels F, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne Interscore Comparison study. J Clin Oncol. 2013;31:944–51.
Yanik GA, Parisi MT, Shulkin BL, Naranjo A, Kreissman SG, London WB, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s Oncology Group. J Nucl Med. 2013;54:541–8.
Li YJ, Dai YL, Cheng YS, Zhang WB, Tu CQ. Positron emission tomography (18)F-fluorodeoxyglucose uptake and prognosis in patients with bone and soft tissue sarcoma: a meta-analysis. Eur J Surg Oncol. 2016;42:1103–14.
Marinelli B, Espinet-Col C, Ulaner GA, McArthur HL, Gonen M, Jochelson M, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am J Nucl Med Mol Imaging. 2016;24:120–7.
Tamandl D, Ta J, Schmid R, Preusser M, Paireder M, Schoppmann SF, et al. Prognostic value of volumetric PET parameters in unresectable and metastatic esophageal cancer. Eur J Radiol. 2016;85:540–5.
Apostolova I, Steffen IG, Wedel F, Lougovski A, Marnitz S, Derlin T, et al. Asphericity of pretherapeutic tumour FDG uptake provides independent prognostic value in head-and-neck cancer. Eur Radiol. 2014;24:2077–87.
Apostolova I, Rogasch J, Buchert R, Wertzel H, Achenbach HJ, Schreiber J, et al. Quantitative assessment of the asphericity of pretherapeutic FDG uptake as an independent predictor of outcome in NSCLC. BMC Cancer. 2014;14:896. doi:10.1186/1471-2407-14-896.
Wetz C, Apostolova I, Steffen IG, Hofheinz F, Furth C, Kupitz D, et al. Predictive value of asphericity in pretherapeutic [111In]DTPA-octreotide SPECT/CT for response to peptide receptor radionuclide therapy with [177Lu]DOTATATE. Mol Imaging Biol. 2017;19:437–45.
European Association of Nuclear Medicine. Dosage card (Version 5.7.2016). Vienna: European Association of Nuclear Medicine; 2016.
Hofheinz F, Pötzsch C, Oehme L, Beuthien-Baumann B, Steinbach J, Kotzerke J, et al. Automatic volume delineation in oncological PET. Evaluation of a dedicated software tool and comparison with manual delineation in clinical data sets. Nuklearmedizin. 2012;51:9–16.
Hughes M, Marsden HB, Palmer MK. Histologic patterns of neuroblastoma related to prognosis and clinical staging. Cancer. 1974;34:1706–11.
Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87.
Mattay KK, Edeline V, Lumbroso J, Tanguy ML, Asselain B, Zucker JM, et al. Correlation of early metastatic response by123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–91.
Fendler WP, Wenter V, Thornton HI, Ilhan H, von Schweinitz D, Coppenrath E, et al. Combined scintigraphy and tumor marker analysis predicts unfavorable histopathology of neuroblastic tumors with high accuracy. PLoS One. 2015;10:e0132809.
Berthold F, Hunnemann DH, Harms D, Käser H, Zieschang J. Serum vanillylmandelic acid/homovanillic acid contributes to prognosis estimation in patients with localised but not with metastatic neuroblastoma. Eur J Cancer. 1992;28A:1950–4.
Cangemi G, Reggiardo G, Barco S, Barbagallo L, Conte M, D’Angelo P, et al. Prognostic value of ferritin, neuron-specific enolase, lactate dehydrogenase, and urinary and plasmatic catecholamine metabolites in children with neuroblastoma. Onco Targets Ther. 2012;5:417–23.
Strenger V, Kerbl R, Dornbusch HJ, Ladenstein R, Ambros PF, Ambros IM, et al. Diagnostic and prognostic impact of urinary catecholamines in neuroblastoma patients. Pediatr Blood Cancer. 2007;48:504–9.
Zambrano E, Reyes-Múgica M. Hormonal activity may predict aggressive behavior in neuroblastoma. Pediatr Dev Pathol. 2002;5:190–9.
Riley RD, Heney D, Jones DR, Sutton AJ, Lambert PC, Abrams KR, et al. A systematic review of molecular and biological tumor markers in neuroblastoma. Clin Cancer Res. 2004;10:4–12.
Acknowledgments
The authors thank Dr. Ingo Steffen for his advice on statistical methodology and Mr. René Höhne for his support in obtaining patient data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures were in accordance with the ethical standards of the respective institutional ethics commissions (vote, EA2/150/16; MV05/11) and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
ROC curves for ASP, MTV and HVA/C regarding the occurrence of an event (GIF 54 kb)
Rights and permissions
About this article
Cite this article
Rogasch, J.M.M., Hundsdoerfer, P., Furth, C. et al. Individualized risk assessment in neuroblastoma: does the tumoral metabolic activity on 123I-MIBG SPECT predict the outcome?. Eur J Nucl Med Mol Imaging 44, 2203–2212 (2017). https://doi.org/10.1007/s00259-017-3786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3786-1