Abstract
Purpose
Triple-negative breast cancer has a poor prognosis. We evaluated several metabolic and volumetric parameters from preoperative 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) in the prognosis of triple-negative breast cancer and compared them with current clinicopathologic parameters.
Methods
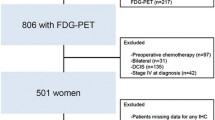
A total of 228 patients with triple-negative breast cancer (mean age 47.0 ± 10.8 years, all women) who had undergone preoperative PET/CT were included. The PET/CT metabolic parameters evaluated included maximum, peak, and mean standardized uptake values (SUVmax, SUVpeak, and SUVmean, respectively). The volumetric parameters evaluated included metabolic tumor volume (MTV) and total lesion glycolysis (TLG). Metabolic and volumetric parameters were evaluated separately for tumor (T) and lymph nodes (N). The prognostic value of these parameters was compared with that of clinicopathologic parameters.
Results
All lymph node metabolic and volumetric parameters showed significant differences between patients with and without recurrence. However, tumor metabolic and volumetric parameters showed no significant differences. In a univariate survival analysis, all lymph node metabolic and volumetric parameters (SUVmax-N, SUVpeak-N, SUVmean-N, MTV-N, and TLG-N; all P < 0.001), T stage (P = 0.010), N stage (P < 0.001), and TNM stage (P < 0.001) were significant parameters. In a multivariate survival analysis, SUVmax-N (P = 0.005), MTV (P = 0.008), and TLG (P = 0.006) with TNM stage (all P < 0.001) were significant parameters.
Conclusions
Lymph node metabolic and volumetric parameters were significant predictors of recurrence in patients with triple-negative breast cancer after surgery. Lymph node metabolic and volumetric parameters were useful parameters for evaluating prognosis in patients with triple-negative breast cancer by 18F-FDG PET/CT, rather than tumor parameters.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18:123–33.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67.
Groheux D, Cochet A, Humbert O, Alberini J-L, Hindié E, Mankoff D. 18F-FDG PET/CT for staging and restaging of breast cancer. J Nucl Med. 2016;57:17S–26S.
Groheux D, Mankoff D, Espié M, Hindié E. 18F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl Med Mol Imaging. 2016;43:983–93.
Jo I, Zeon SK, Kim SH, Kim HW, Kang SH, Kwon SY, et al. Correlation of primary tumor FDG uptake with clinicopathologic prognostic factors in invasive ductal carcinoma of the breast. Nucl Med Mol Imaging. 2015;49:19–25.
Groheux D, Hindié E, Delord M, Giacchetti S, Hamy A-S, de Bazelaire C, et al. Prognostic impact of 18FDG-PET-CT findings in clinical stage III and IIB breast cancer. J Natl Cancer Inst. 2012;19:1879–87.
Chang JS, Lee J, Kim HJ, Kim KH, Yun M, Kim SI, et al. 18F-FDG/PET may help to identify a subgroup of patients with T1-T2 breast cancer and 1–3 positive lymph nodes who are at a high risk of recurrence after mastectomy. Cancer Res Treat. 2016;48:508–17.
Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters. Cancer. 2008;112:995–1000.
Groheux D, Giacchetti S, Moretti J-L, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Vicente AMG, Castrejón ÁS, Martín AL, López-Muñiz IC, Madero VM, Sánchez MMM, et al. Molecular subtypes of breast cancer: metabolic correlation with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1304–11.
Marinelli B, Espinet-Col C, Ulaner GA, McArthur HL, Gonen M, Jochelson M, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am J Nucl Med Mol Imaging. 2016;6:120–7.
Hyun SH, Ahn HK, Park YH, Im Y-H, Kil WH, Lee JE, et al. Volume-based metabolic tumor response to neoadjuvant chemotherapy is associated with an increased risk of recurrence in breast cancer. Radiology. 2014;275:235–44.
Son SH, Lee S-W, Jeong SY, Song B-I, Chae YS, Ahn B-C, et al. Whole-body metabolic tumor volume, as determined by 18F-FDG PET/CT, as a prognostic factor of outcome for patients with breast cancer who have distant metastasis. AJR Am J Roentgenol. 2015;205:878–85.
Kim J, Yoo SW, Kang S-R, Cho S-G, Oh J-R, Chong A, et al. Prognostic significance of metabolic tumor volume measured by 18F-FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46:278–85.
Song B-I, Lee S-W, Jeong SY, Chae YS, Lee WK, Ahn B-C, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med. 2012;53:1337–44.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:e48–72.
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43.
Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862.
Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, et al. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med. 2013;54:5–11.
Groheux D, Hindié E, Giacchetti S, Delord M, Hamy A-S, de Roquancourt A, et al. Triple-negative breast cancer: early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nucl Med. 2012;53:249–54.
Groheux D, Giacchetti S, Delord M, de Roquancourt A, Merlet P, Hamy A, et al. Prognostic impact of 18F-FDG PET/CT staging and of pathological response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:377–85.
Humbert O, Riedinger J-M, Charon-Barra C, Berriolo-Riedinger A, Desmoulins I, Lorgis V, et al. Identification of biomarkers including 18FDG-PET/CT for early prediction of response to neoadjuvant chemotherapy in triple-negative breast cancer. Clin Cancer Res. 2015;21:5460–8.
Koo HR, Park JS, Kang KW, Han W, Park IA, Moon WK. Correlation between 18F-FDG uptake on PET/CT and prognostic factors in triple-negative breast cancer. Eur Radiol. 2015;25:3314–21.
Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N, et al. Utility of 18F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat. 2015;150:209–17.
Yue Y, Cui X, Bose S, Audeh W, Zhang X, Fraass B. Stratifying triple-negative breast cancer prognosis using 18F-FDG-PET/CT imaging. Breast Cancer Res Treat. 2015;153:607–16.
Lucignani G. SUV and segmentation: pressing challenges in tumour assessment and treatment. Eur J Nucl Med Mol Imaging. 2009;36:715–20.
Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–4.
Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–22.
Chen HH, Chiu N-T, Su W-C, Guo H-R, Lee B-F. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non–small cell lung cancer. Radiology. 2012;264:559–66.
Pak K, Cheon GJ, Nam H-Y, Kim S-J, Kang KW, Chung J-K, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–90.
Ferguson DJ, Meier P, Karrison T, Dawson PJ, Straus FH, Lowenstein FE. Staging of breast cancer and survival rates: an assessment based on 50 years of experience with radical mastectomy. JAMA. 1982;248:1337–41.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.
Liao C-T, Chang JT-C, Wang H-M, Ng S-H, Hsueh C, Lee L-Y, et al. Preoperative [18F]fluorodeoxyglucose positron emission tomography standardized uptake value of neck lymph nodes predicts neck cancer control and survival rates in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys. 2009;74:1054–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2009-0093820), and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1072).
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kim, Yi., Kim, Y.J., Paeng, J.C. et al. Prediction of breast cancer recurrence using lymph node metabolic and volumetric parameters from 18F-FDG PET/CT in operable triple-negative breast cancer. Eur J Nucl Med Mol Imaging 44, 1787–1795 (2017). https://doi.org/10.1007/s00259-017-3748-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3748-7